Cardio-protective role of ranolazine during percutaneous coronary intervention in chronic coronary syndrome patients
ORIGINAL RESEARCH ARTICLE
Cardio-protective role of ranolazine during percutaneous coronary intervention in chronic coronary syndrome patients
Article Summary
- DOI: 10.24969/hvt.2025.566
- CARDIOVASCULAR DISEASES
- Published: 21/05/2025
- Received: 19/04/2025
- Revised: 13/05/2025
- Accepted: 13/05/2025
- Views: 3949
- Downloads: 1679
- Keywords: Cardiac Troponin I, cardio-protection, chronic coronary syndrome, PCI-related myocardial injury, ranolazine
Address for Correspondence: Ahmed G.Bakry. Cardiology Division, Department of Internal Medicine, Qena Faculty of Medicine, South Valley University, Qena, 83523 Egypt
Email: ahmedgbakry@gmail.com Phone: 0201012895996
ORCID: Ahmed G.Bakry - 0000-0002-4581-4927; Hossam Eldin M. Mahmoud - 0000-0003-4973-7639; Areej Alkhateeb
-: 0000-0002-2683-8640; Mohammed H. Hassan - 0000-0003-2698-9438
Facebook: Ahmed G. Bakry - @ahmed.bakry.1806; Hossam Eldin M. Mahmoud - @hosam.ismail.96; Areej Alkhateeb - @areej.cardio; Mohammed H. Hassan - @mohammed.hassaan.731
X (twitter): Ahmed G. Bakry - @AhmedGBakry
Ahmed G.Bakry 1a *, Hossam Eldin M Mahmoud 1a, Areej Alkhateeb 1a, Mohammed H. Hassan 1b, Marwa Abdelhady2
1a Cardiology Division of Internal Medicine Department and 1bDepartment of Medical Biochemistry, Faculty of Medicine,
South Valley University, Qena, 83523 Egypt
2Department of Internal Medicine, Faculty of Medicine, Luxor University, Luxor, Egypt.
Abstract
Objectives: Chronic coronary syndrome (CCS) is a major cause of morbidity and mortality. Despite advances in percutaneous coronary intervention (PCI), patients remain at risk for PCI-related myocardial injury (PMI). The best pharmacological strategy to reduce PMI in high-risk patients is still unclear.
This study evaluates the cardio-protective role of ranolazine in reducing PMI in CCS patients, focusing on cardiac troponin I (cTnI) levels.
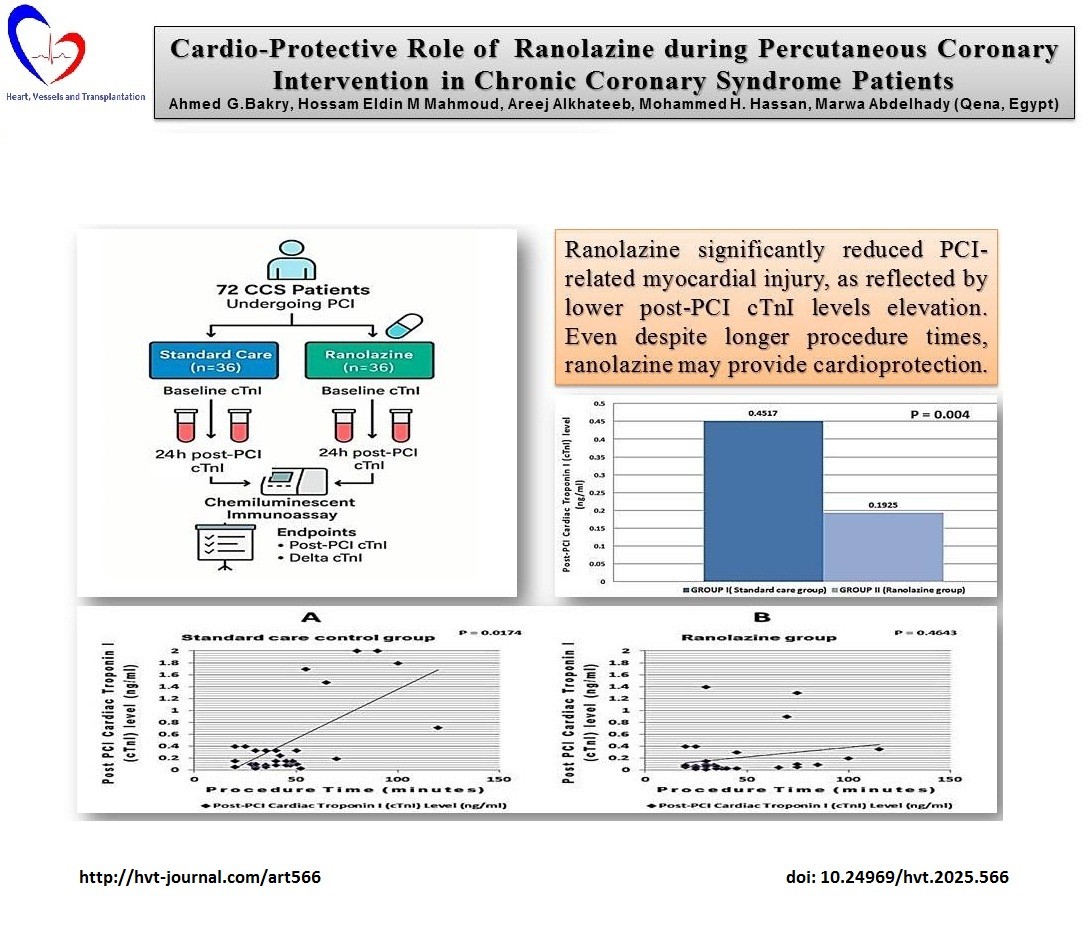
Methods: A prospective observational study enrolled 72 CCS patients undergoing PCI, divided into two groups: standard care (Group I, n=36) and ranolazine (Group II, n=36). Serum cTnI levels were measured at baseline and 24 hours post-PCI using chemiluminescent immunoassay. The primary endpoint was post-PCI cTnI comparison, with secondary endpoints including delta change in cTnI levels, cTnI elevation above the upper reference limit (URL), and correlation between procedure time and post-PCI cTnI levels.
Results: The ranolazine group showed a significant reduction in post-PCI cTnI levels compared to the standard care group (0.1925 (0.33) vs. 0.4517 (0.66) ng/mL, p=0.004). The delta change in cTnI was also lower in the ranolazine group (0.1531 (0.33) vs. 0.4128 (0.66) ng/mL, p=0.005). Fewer patients in the ranolazine Group had cTnI levels above the URL (9 vs. 19, p=0.029) as compared to standard care group. A significant positive correlation between procedure time and cTnI levels was found in the standard care group but not in the ranolazine group.
Conclusion: Ranolazine reduced PCI-related myocardial injury, suggesting its potential as an adjunct therapy for CCS patients. Further research is needed to confirm its clinical efficacy and long-term benefits.
Key words: Cardiac Troponin I, cardio-protection, chronic coronary syndrome, PCI-related myocardial injury, ranolazine
Introduction
Chronic coronary syndrome (CCS) is a major cause of morbidity and mortality due to progressive coronary artery disease (CAD) and persistent ischemia. Despite advances in medical therapy and percutaneous coronary intervention (PCI), patients remain at high risk for recurrent ischemia, myocardial infarction, and heart failure. While PCI restores blood flow, it also poses a risk of PCI-related myocardial injury (PMI) due to ischemia-reperfusion injury, microvascular dysfunction, and endothelial damage, which can lead to long-term myocardial dysfunction (1, 2).
Graphical abstract
Elevated cardiac troponin I (cTnI) is a key biomarker for diagnosing PMI. According to the Fourth Universal Definition of Myocardial Infarction (3), PMI is defined by a cTnI increase >1× URL in patients with normal baseline levels. Higher cTnI levels are associated with worse PCI outcomes, including increased risk of cardiovascular events and heart failure. The ESC Working Group and EAPCI provide guidance on managing periprocedural myocardial injury and type 4a myocardial infarction (MI) in CCS patients undergoing PCI.
Minor myocardial injury (cTnI >1x and <5x URL) is common but not linked to major adverse cardiac events (MACE). Major injury (>5x URL without ischemic evidence) occurs in <20% and raises 1-year mortality risk, while type 4a MI (>5x URL with ischemic evidence) increases MACE risk at 30 days and 1 year. Mechanisms include side-branch occlusion, embolization, and endothelial dysfunction, with risk factors such as age, comorbidities, lesion complexity, and procedural factors.
These findings underscore the importance of preventing PMI to improve outcomes, especially in high-risk CCS patients undergoing PCI (1, 2 , 4).
Ranolazine hydrochloride, approved by the FDA in January 2006 for treating chronic stable angina, is an anti-anginal agent. Beyond angina relief, it offers benefits in managing arrhythmias, particularly atrial fibrillation, as well as diastolic dysfunction, pulmonary hypertension, chemotherapy-induced cardiotoxicity, and diabetes (5, 6). Ranolazine is a piperazine derivative typically dosed at 500–1000 mg twice daily. Its plasma levels peak 2–5 hours after oral administration, with a 2-hour elimination half-life. The drug is metabolized in the liver and excreted through the kidneys (7). Ranolazine works by blocking late sodium channels (INa), reducing calcium buildup, decreasing left ventricular wall tension, improving coronary blood flow, and providing angina relief (8). Additionally, it stabilizes myocardial cell membranes and inhibits the late rectifier potassium current, contributing to its anti-arrhythmic effects. In diabetes, it is thought to block sodium channels in pancreatic islet alpha cells, reducing glucagon release and preserving beta-cell function (5, 9, 10).
The 2024 ESC Guidelines for the Diagnosis and Treatment of CCS and the 2023 ACC/AHA guidelines for the management of patients with chronic CAD recommend ranolazine as a second-line therapy for stable CAD patients with persistent angina despite optimal medical therapy, including beta-blockers and calcium channel blockers (11-13).
The ideal pharmacotherapy to reduce future cardiac events in patients with post-PCI major injury and type 4 MI remains unclear (14). Ranolazine may reduce myocardial injury during PCI. It blocks the late sodium current in heart cells. This action lowers calcium overload and improves the heart's oxygen balance. It does not change heart rate or blood pressure. It suits patients who cannot take other anti-anginal drugs (15, 16).
This study aims to explore the cardio-protective role of ranolazine during PCI in CCS patients, focusing on its effect on reducing PCI-related myocardial injury, as measured by cTnI levels. The goal is to optimize PCI outcomes and provide further insights into therapeutic strategies for CCS patients.
Methods
Study design and population
This was a prospective observational study conducted at a single center within the cardiology division of the Department of Internal Medicine, South Valley University Hospital, Qena, Egypt during the study period from 1st of May, 2024 to 30th of January, 2025. Seventy-two patients with CCS and Class I indications for PCI, due to persistent symptoms or a positive stress test, were enrolled. This study was conducted in accordance with the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines.
Patient selection
In our study, 100 patients were initially assessed for eligibility. Of these, 28 patients were excluded due to the following reasons: elevated baseline cTnI levels (n = 10), acute coronary syndrome (n = 8), advanced renal impairment (n = 10). The remaining 72 patients were included and predefined into two groups:
• Group I (Standard care group): 36 patients receiving conventional PCI treatment without any additional medication.
• Group II (Ranolazine group): 36 patients receiving ranolazine as an adjunct to standard PCI therapy.
Inclusion criteria: The study included adult patients (aged 18-80) with CCS, as defined by the 2024 European Society of Cardiology (ESC) guidelines (13), and Class I indications for PCI due to persistent symptoms or a positive stress test. Eligible patients had documented CAD with significant stenosis (≥70% luminal narrowing in one or more coronary arteries), and were scheduled for elective PCI.
Exclusion criteria: Patients with acute coronary syndromes within 3 months, elevated baseline cardiac enzymes, prolonged QT interval, use of QT-prolonging or CYP3A4-inhibiting drugs, left ventricular ejection fraction (LVEF) <40%, advanced renal impairment (eGFR <60 mL/min/1.73 m²), elevated liver enzymes, chronic liver or muscle disease, prior ranolazine use, hypersensitivity to ranolazine, pregnancy, breastfeeding, or participation in other interventional studies were excluded.
Ethical considerations: The study protocol was approved by the Faculty of Medicine, South Valley University Ethics Committee on Research Involving Humans (Ethical approval code: SVU-MED-MBC004-4-24-12-1024), approval date was April,2024. The study adhered to the ethical guidelines of the 1975 Declaration of Helsinki, and all participants provided written informed consent prior to enrollment.
Baseline Variables
We collected demographic (age, sex), anthropometric (body mass index (BMI)), and clinical risk factors (diabetes, hypertension, smoking, dyslipidemia) for all patients. Clinical variables included LVEF and serum creatinine. Coronary angiography variables encompassed the extent of coronary artery disease (CAD), defined as the number of vessels with ≥70% stenosis, target vessel (left anterior descending (LAD), left circumflex (LCX), right coronary artery (RCA)), presence of chronic total occlusion (CTO) lesions, and multivessel intervention. These variables were recorded to characterize the study population and ensure comparability between groups.
Intervention and Procedures
Group I (Standard care group) received standard of care for PCI without ranolazine, which includes antiplatelet therapy (aspirin and P2Y12 inhibitors), other CCS therapy (statins and beta blockers or CCB), renin-angiotensin-aldosterone system (RAAS) inhibitors (e.g., angiotentsin-converting enzyme inhibitors (ACEI) or angiotensin receptor blockers (ARBs)) in eligible patients and standard peri-procedural medications (heparin and nitrates).
Group II (Ranolazine group) received ranolazine (750 mg twice daily) in addition to standard PCI treatment. Ranolazine was administered orally, starting 7 days prior to the PCI procedure and continued for 48 hours post-PCI. Patients instructed to report any adverse events, including those related to ranolazine administration (e.g., dizziness, nausea, arrhythmias).
Percutaneous coronary intervention
The PCI procedure followed standard practices, with operators selecting the appropriate coronary access and stenting strategy based on the patient's clinical condition and coronary anatomy according to the 2018 ESC/EACTS guidelines on myocardial revascularization (17). Procedural success was achieved with TIMI grade 3 flow in the target vessel, no significant side branch occlusion, no major electrocardiographic changes, and no periprocedural complications, including hemodynamic instability or acute MI. The procedural details were recorded and after the intervention, all hemostatic measures were taken for the patient’s safety.
Serum cardiac troponin I assay
Serum cTnI levels were measured at baseline pre-PCI and 24 hours post-PCI. After withdrawal of 5 mL of venous blood from each participant into serum gel separator tubes, samples were immediately stored at room temperature (18–25°C) for a maximum of 30 minutes prior to centrifugation. Blood samples were centrifuged at 3500 rpm for 15 minutes to separate the serum. The separated serum was promptly aliquoted and stored at −80°C until analysis to ensure biomarker stability and minimize degradation. CTnI was analyzed using the Chemiluminescent Microparticle Immunoassay (CMIA) on the Architect i2000 platform (Abbott Diagnostics, USA). The assay has a dynamic range of 0.01–50 ng/mL. All assays were performed in accordance with the manufacturer’s guidelines, including the use of quality control samples to validate assay accuracy and reproducibility. These procedures were implemented to ensure the reliability and reproducibility of the biomarker data across all samples. The upper reference limit (URL) for cTnI was (0.1 ng/ml) as in our previously published work (18, 19).
Endpoints
The primary endpoint of the study was the cTnI level at 24 hours post-PCI, which serves as a biomarker for myocardial injury. Secondary endpoints included:
1. Delta change in cTnI levels (difference between pre-PCI and post-PCI levels).
2. CTnI elevation above the URL.
3. Correlation between procedure time and post-PCI cTnI levels.
Sample size
The study enrolled 72 patients (36 per group), selected based on an estimated effect size of ranolazine in reducing post-PCI cTnI levels, with a significance level of 0.05 and 80% power (Cohen’s d = 0.67) which was considered clinically meaningful, consistent with prior studies investigating cardioprotective interventions.
Of 100 patients initially screened, 28 were excluded due to elevated baseline cTnI (n = 10), acute coronary syndrome (n = 8), or advanced renal disease (n = 10). The final 72 patients completed the study protocol without dropouts.
Statistical analysis
Statistical analysis was performed using GraphPad InStat 3.10 software (GraphPad Software, San Diego, CA, USA). The Shapiro-Wilk and Kolmogorov-Smirnov (KS) test were used to quantitatively test the normality of the data after it had been visually evaluated using histograms and a common Q-Q plot. Continuous variables are expressed as mean (standard deviation, SD). Descriptive statistics will be used to summarize patient demographics and baseline characteristics (mean (SD) for continuous variables, frequency and percentage for categorical variables). The primary analysis assessed the change in cTnI levels from baseline to 24 hours post-PCI between the ranolazine and the standard care groups using an independent t-test or Mann-Whitney U test depending on the normality of data. The difference between values before and after an intervention is known as the delta change (Δ). Pearson or Spearman correlation coefficient was used to assess the relationship between procedure time and post-PCI cTnI levels in both groups depending on the normality of data. Multiple regression analysis was performed to examine the relationship between post-PCI cTnI delta change and key procedural and clinical variables, including the number of stents, procedure time, age, and LVEF. The model assessed the combined effect of these variables on cTnI changes. A p-value of < 0.05 was considered statistically significant and highly significant at p < 0.01.
Results
Patient demographic, clinical and procedural characteristics
The baseline demographic, clinical and procedural characteristics of the ranolazine and control groups are summarized in Tables 1 and 2.
|
Table 1. Continuous clinical and procedural variables in the standard care and ranolazine groups |
||||
|
Variables |
Standard care (n=36) |
Ranolazine (n=36) |
Test statistic value (t or U) |
p |
|
Age, years |
60.44 (7.79) |
57.78 (7.58) |
t = 1.472 |
0.15 |
|
BMI, kg/m² |
27.08 (2.56) |
27.31 ( 2.31) |
t = 0.385 |
0.70 |
|
LVEF, % |
54.9 (6.44) |
55.8 (7.79) |
t = 0.527 |
0.59 |
|
Serum creatinine, mg/dl |
1.04 (0.22) |
1.08 (0.24) |
t = 0.760 |
0.45 |
|
Procedure time, minutes |
45.53 (22.54) |
41.89 (24.19) |
U = 510 |
0.12 |
|
Mean stents per patient, n |
1.75 (0.77) |
1.67 (0.79) |
U = 603 |
0.62 |
|
Predilations performed, n |
2.4 (2.0) |
2.7 (2.8) |
t = 0.48 |
0.63 |
|
Contrast, ml |
258.33 (43.92) |
241.67 (59.16) |
t = 1.20 |
0.23 |
|
Radiation dose, mgy |
4072.4 (1105) |
4055.7 (1751) |
t = 0.05 |
0.96 |
|
Baseline cTnI, ng/ml |
0.0388 (0.009) |
0.0394 (0.010) |
U = 632 |
0.86 |
|
cTnI 24hr Post PCI, ng/ml |
0.4517 (0.6618) |
0.1925 (0.3305) |
U = 389 |
0.004* |
|
cTnI delta change post PCI |
0.4128 (0.6644) |
0.1531 (0.3332) |
U = 396 |
0.005* |
|
Continuous data represented as mean (SD). *Significant p value is < 0.05 Independent t-test (t) or Mann-Whitney U test (U) depending on the normality of data BMI - body mass index, cTnI - cardiac troponin I, LVEF - left ventricular ejection fraction, n – number, % - percentage, PCI - percutaneous coronary intervention, SD - standard deviation |
||||
Statistical analysis showed no significant differences between the two groups in terms of age (p = 0.15), sex (p = 0.34 ), BMI (p= 0.70), diabetes (p = 0.63), hypertension (p = 0.64), smoking (p = 0.47), dyslipidemia (p = 1.00), LVEF (p= 0.59), and serum creatinine (p = 0.45).
|
Table 2. Categorical clinical and procedural variables in the standard care and ranolazine groups |
||||||
|
Variables |
Standard Care (N=36) |
Ranolazine (N=36) |
p |
|||
|
Sex |
Male , n (%) |
18 (50) |
23 (64) |
0.34 |
||
|
Female , n (%) |
18 (50) |
13 (36) |
||||
|
Diabetes mellitus , n (%) |
16 (44) |
13 (36) |
0.63 |
|||
|
Hypertension , n (%) |
17 (47) |
20 (56) |
0.64 |
|||
|
Smoking , n (%) |
12 (33) |
16 (44) |
0.47 |
|||
|
Dyslipidemia , n (%) |
20 (56) |
19 (52) |
1.00 |
|||
|
Previous PCI , n (%) |
10 (28) |
6 (17) |
0.74 |
|||
|
Previous MI , n (%) |
8 (22) |
11 (30) |
0.59 |
|||
|
Target vessel |
LAD , n (%) |
28 (77) |
24 (66) |
0.43 |
||
|
LCX , n (%) |
7 (19) |
15 (41) |
0.07 |
|||
|
RCA , n (%) |
12 (33) |
13 (36) |
1.00 |
|||
|
CTO Lesions , n (%) |
6 (16) |
4 (11) |
0.73 |
|||
|
Multivessel intervention, n (%) |
13 (36) |
14 (38) |
1.00 |
|||
|
DES implantation, n (%) |
35 (97) |
35 (97) |
1.0000 |
|||
|
DCB only , n (%) |
1 (3) |
1 (3) |
1.00 |
|||
|
Post dilation performed , n (%) |
17 (47) |
14 (39) |
0.64 |
|||
|
cTnI any elevation > URL , n (%) |
19 (52) |
9 (25) |
0.029* |
|||
|
Standard medical treatment, n (%) |
Aspirin |
36 (100) |
36 (100) |
1.00 |
||
|
P2Y12 inhibitors |
36 (100) |
36 (100) |
1.00 |
|||
|
Statins |
34 (94.4) |
33 (91.7) |
0.87 |
|||
|
Beta-blockers |
30 (83.3) |
29 (80.6) |
0.76 |
|||
|
Calcium channel blockers |
10 (27.8) |
11 (30.6) |
0.80 |
|||
|
RAAS inhibitors |
22 (61.1) |
20 (55.6) |
0.64 |
|||
|
Data are presented as number (n) and percentage (%). *Significant p- value is < 0.05. Fisher's exact test was used for all categorical reference limit variables cTnI - cardiac troponin I, CTO - chronic total occlusion, DCB – drug-coated balloon, DES –drug-eluting stent, LAD - left anterior descending coronary artery, LCX - left circumflex coronary artery, MI - myocardial infarction, n - number, %, percentage, PCI - percutaneous coronary intervention, RAAS – renin-angiotensin –aldosterone system, RCA, - right coronary artery, URL - upper reference level |
||||||
There were no significant differences between the two groups in procedural variables such as procedure time (p = 0.12), contrast usage (p = 0.23), radiation dose (p = 0.96), the mean stents per patient (p = 0.62), target vessel revascularization (p > 0.05), CTO lesions (p = 0.73), multivessel intervention (p = 1.00), predilations and postdilations performed (p = 0.6).
Baseline medication use, including aspirin, P2Y12 inhibitors, statins, beta-blockers, calcium channel blockers, and RAAS inhibitors, was comparable between both groups (p = 0.87 for statins, p > 0.05 for others). No adverse events were reported in both groups. This indicates that the two groups were well-matched at baseline.
Cardiac troponin I (cTnI) levels
Baseline mean cTnI levels were comparable between the ranolazine and control groups (0.0394 (0.010) ng/mL vs. 0.0388 (0.009), p = 0.86). There was a statistically significant difference in the 24-hour post-PCI cTnI levels between the two groups as shown in Table 1 and Figure 1. The Standard Care Group had a mean cTnI level of 0.4517 (0.6618) ng/mL, while the ranolazine group had a significantly lower mean level of 0.1925 (0.330) ng/mL (p = 0.004). These findings indicate a reduced myocardial injury in the Ranolazine group, highlighting its potential cardioprotective effect.
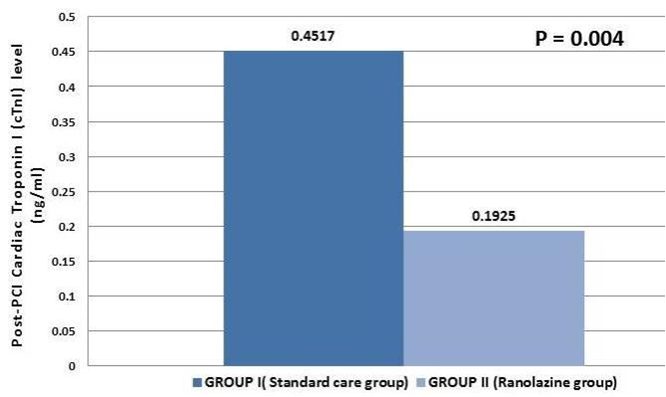
Figure 1. Cardiac troponin I (cTnI) level 24 hours post PCI. There was a significant difference in 24-hour post-PCI cTnI levels between the two groups (p=0.004), suggesting reduced myocardial injury and a potential cardioprotective effect of Ranolazine.
PCI - percutaneous coronary intervention
The observed reduction in post-PCI cTnI levels appeared to be independent of procedural or clinical factors, further supporting the cardioprotective role of ranolazine.
The delta change in cTnI levels (difference between baseline and 24-hour post-PCI values) also demonstrated a statistically significant difference between the groups. The standard care group had a mean delta change of 0.4128 (0.6644) ng/mL, whereas the ranolazine group had a significantly smaller change of 0.1531 (0.3332) ng/mL (p = 0.005), (Table.1). This further reinforces the cardioprotective potential of ranolazine, as it results in a smaller increase in cTnI post-PCI.
The incidence of cTnI elevation above the URL post-PCI was significantly lower in the ranolazine group compared to the standard care group. In the standard care group, 19 patients had a cTnI level above the URL, while only 9 patients in the ranolazine group exceeded the threshold (p = 0.029), (Table.2). This suggests that ranolazine may play a role in reducing the extent of myocardial injury after PCI.
Correlation between procedure time and Post-PCI cTnI levels
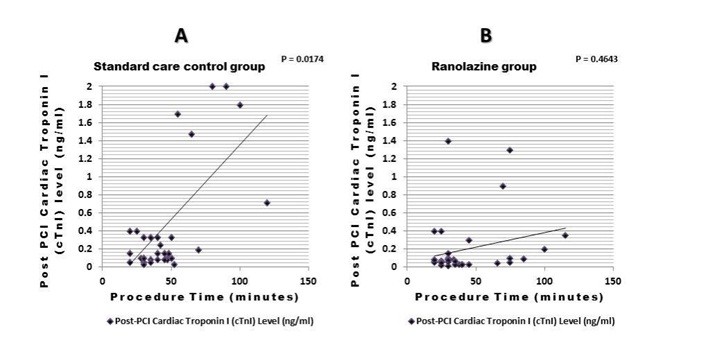
A significant positive correlation was observed between procedure time and post-PCI cTnI levels in the standard care group (correlation coefficient = 0.0174). However, in the ranolazine group, the correlation was non-significant (correlation coefficient = 0.4643). This finding denotes that, despite longer procedure times, ranolazine may provide cardioprotection, alleviating the expected increase in myocardial injury associated with prolonged PCI procedures as shown in (Fig. 2).
![]()
Figure 2. Correlation between procedure time (minutes) and post-PCI cTnI Levels (ng/ml).
(A) A significant positive correlation between procedure time and post-PCI cTnI levels in the standard care control group (correlation coefficient = 0.0174). (B) No significant correlation in the ranolazine group (p = 0.46), suggesting that ranolazine may reduce the impact of prolonged procedure time on myocardial injury.
Spearman correlation analysis
PCI - percutaneous coronary intervention
Determinants of myocardial injury post-PCI
Multiple regression analysis examined the relationship between post-PCI cTnI delta change and key procedural and clinical variables, including the number of stents, procedure time, age, and LVEF. The model was statistically significant (p = 0.0063) with an R² of 19.0%, indicating that these variables together explained 19% of the variance in cTnI changes. However, procedure time was the only significant predictor (β = 0.0116, 95% CI: 0.0036 to 0.0197, p = 0.005), showing a direct association with increased cTnI levels. The number of stents (p = 0.58), age (p = 0.71), and LVEF (p = 0.89) did not significantly contribute to the model. These findings suggest that longer procedure durations, rather than stent burden or baseline cardiac function, play a more prominent role in determining myocardial injury post-PCI (Table 3).
|
Table 3. Multiple Linear Regression predicting Post-PCI cTnI Delta |
|
|||||||
|
Variable |
Coefficient (β) |
Standard Error |
95% Confidence Interval |
p |
|
|||
|
Intercept |
0.1233 |
0.6533 |
-1.182 to 1.428 |
0.85 |
||||
|
Number of Stents |
-0.0670 |
0.1193 |
-0.3053 to 0.1713 |
0.58 |
||||
|
Procedure Time |
0.0116 |
0.0040 |
0.0036 to 0.0197 |
0.005* |
||||
|
Age |
-0.0029 |
0.0077 |
-0.0183 to 0.0126 |
0.71 |
||||
|
LVEF |
-0.0011 |
0.0086 |
-0.0183 to 0.0161 |
0.89 |
||||
|
Model Summary:R² = 0.190 Adjusted R² = 0.1416 F(4,67) = 3.928 P = 0.0063 n = 72. *Significant p value is < 0.05. cTnI, cardiac troponin I, LVEF - left ventricular ejection fraction, n- number, PCI - percutaneous coronary intervention |
||||||||
Discussion
This study assessed ranolazine's cardio-protective effect in CCS patients undergoing PCI. Ranolazine lowered cTnI levels after the procedure. The change in cTnI and the count of patients with levels above the URL were lower with ranolazine. The study shows that ranolazine reduces heart injury during PCI.
Elevated cTnI levels are a well-established marker of myocardial injury, often used to estimate the extent of ischemia and damage following PCI. Previous studies have indicated that myocardial injury during PCI, even in the absence of clinical symptoms of infarction, can be associated with poor long-term outcomes, including heart failure and arrhythmias (20-23). In this context, our findings support the potential role of ranolazine in reducing myocardial injury, consistent with prior research. In a randomized trial by Pelliccia et al., ranolazine (1,000 mg twice daily for 7 days) reduced myocardial injury in 70 stable angina patients undergoing elective PCI. The ranolazine group showed lower myocardial infarction rates (6% vs. 22%, p=0.041) and significantly lower postprocedural creatine kinase MB and cTnI levels (both p<0.05), with no significant adverse effects. This supports ranolazine's potential to alleviate ischemia and myocardial injury during PCI (24).
Our study found a drop in cTnI levels with ranolazine. This suggests that it protects heart cells by reducing ischemic injury or its metabolic effects. Ranolazine lowers intracellular calcium overload during ischemia. This action may reduce heart injury and improve PCI outcomes. This mechanism is supported by both animal models and clinical studies. Zacharowski et al. (25) demonstrated for the first time that ranolazine significantly reduces infarct size and cardiac troponin T release in rats after coronary artery occlusion-reperfusion. It achieves this by inhibiting fatty acid beta-oxidation, lowering acetyl-CoA levels, and activating pyruvate dehydrogenase, leading to more efficient adenosine tri-phosphate production, reduced lactic acid buildup, and improved heart function under reduced oxygen supply (25).
Furthermore, the study by Iqbal et al. (26) involved 110 patients with chronic stable angina undergoing elective PCI. Patients were randomized to receive ranolazine (1,000 mg twice daily for 7 days, n=55) or a control group (n=55). The ranolazine group experienced less periprocedural myocardial injury and lower PCI-related myocardial infarction. Post-procedural cardiac marker levels were significantly reduced in the ranolazine group. No significant adverse effects were observed. This study supports ranolazine’s cardioprotective role in elective PCI and complements our findings, further validating its ability to reduce myocardial injury through its unique ion channel-modifying properties (26).
The study found a link between procedure time and cTnI levels in the standard care group (P = 0.017). Longer procedures relate to myocardial injury. The ranolazine group shows no link (P = 0.46). These findings align with previous research, emphasizing procedure time as a key determinant of myocardial injury, while the number of stents, age, and LVEF had minimal impact. Also, avoiding prolonged balloon inflation help to reduce post-PCI troponin elevations. This supports the need for strategies to optimize PCI duration and highlights ranolazine’s potential role in reducing ischemic injury in high-risk interventions (1).
Our results match previous trials on ranolazine in PCI. Kourtis et al. (27) studied 150 patients scheduled for nonemergent PCI. They split patients into three groups: control, RIPC (Preconditioning before PCI), and ranolazine. The ranolazine group had lower cTnI up to 24 hours, lower creatine phosphokinase at 4, 10, and 24 hours, and lower creatine kinase –MB level at 10 hours. This suggests that RIPC with ranolazine lowers ischemia and myocardial enzyme levels, further supporting its cardioprotective role (27).
Also, regarding studies investigating other adjunctive therapies such as nicorandil, a meta-analysis of 14 studies with 1,762 patients compared nicorandil to control during PCI. Nicorandil lowered the risk of periprocedural myocardial infarction (RR 0.73, 95% CI 0.61-0.86) and major adverse cardiovascular events (RR 0.76, 95% CI 0.58-0.99), demonstrating its cardioprotective benefits (28).
While other therapies like statins and ACE inhibitors offer benefits in reducing PMI, similar baseline medication use in both groups indicates that they were well-matched. This suggests ranolazine’s unique mechanism of action may be particularly independently effective in reducing ischemia-related myocardial injury.
Study limitations
While this study provides promising data on the potential of ranolazine as a cardioprotective agent, several limitations must be acknowledged. First, the study was conducted at a single center with a relatively small sample size (n=72), which may limit the generalizability of the results.
Larger trials with multi-biomarker strategy are needed to confirm ranolazine's effects and its impact on long-term outcomes and MACE in PCI patients.
Conclusion
This study shows that ranolazine significantly reduced PCI-related myocardial injury, as reflected by lower post-PCI cTnI levels elevation. These results suggest ranolazine may provide cardioprotective benefits during PCI, particularly in more complex procedures, improving patient outcomes. Further research is needed to confirm these findings, investigate the mechanisms of this protection, and evaluate the long-term effects of ranolazine on clinical outcomes in coronary artery disease management.
Ethics: Cases were collected correspondingly with the guidelines established in the Declaration of Helsinki. This study was approved by the ethics committee of Faculty of Medicine, South Valley University, Qena, Egypt (Ethical approval code: SVU-MED-MBC004-4-24-12-1024). Informed written consent was taken from the included participants for participation in the study.
Peer-review- External and internal
Conflict of interest- None to declare
Authorship: A.G.B, H.E.M.M., A.A., M.H.H., and M.A. conceived and designed the project. A.G.B, H.E.M.M., A.A., M.H.H., and M.A. collected data. A.G.B, H.E.M.M., A.A., M.H.H., and M.A.. analyzed and interpreted the data. M.H.H. performed the laboratory assays. A.G.B. and M.H.H. drafted the manuscript. All authors have read and approved the final manuscript and fulfilled all authorship criteria for publication.
Acknowledgement and Funding: None to declare
Statement on A.I.-assisted technologies use- Authors declare that they did not use AI-assisted technologies in preparation of this manuscript
Availability of data and material: The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
References
| 1.Bulluck H, Paradies V, Barbato E, Baumbach A, Bøtker HE, Capodanno D, et al. Prognostically relevant periprocedural myocardial injury and infarction associated with percutaneous coronary interventions: a Consensus Document of the ESC Working Group on Cellular Biology of the Heart and European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 2021; 42: 2630-42. https://doi.org/10.1093/eurheartj/ehab271 PMid:34059914 PMCid:PMC8282317 |
||||
| 2.Cuculi F, Lim CCS, Banning AP. Periprocedural myocardial injury during elective percutaneous coronary intervention: is it important and how can it be prevented? Heart 2010; 96: 736-40. https://doi.org/10.1136/hrt.2009.186189 PMid:20448123 |
||||
| 3.Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction. Eur Heart J 2019; 40: 237-69. https://doi.org/10.1093/eurheartj/ehy462 PMid:30165617 |
||||
| 4.Li Y, Pei H, Bulluck H, Zhou C, Hausenloy DJ. Periprocedural elevated myocardial biomarkers and clinical outcomes following elective percutaneous coronary intervention: a comprehensive dose-response meta-analysis of 44,972 patients from 24 prospective studies. EuroIntervention 2020; 15: 1444-50. https://doi.org/10.4244/EIJ-D-19-00737 PMid:31829942 |
||||
| 5.Manolis AS, Kallistratos MS, Poulimenos LE. Anti-ischemic and pleiotropic effects of ranolazine in chronic coronary syndromes. Am J Med Sci 2024; 367: 155-9. https://doi.org/10.1016/j.amjms.2023.12.001 PMid:38072070 |
||||
| 6.Mihos CG, Krishna RK, Kherada N, Pineda AM, Santana O. The use of ranolazine in non-anginal cardiovascular disorders: a review of current data and ongoing randomized clinical trials. Pharmacol Res 2016; 103: 49-55. https://doi.org/10.1016/j.phrs.2015.10.018 PMid:26546970 |
||||
| 7.Tamargo J, López-Sendón J. Ranolazine: a better understanding of its pathophysiology and patient profile to guide treatment of chronic stable angina. Future Cardiol 2022; 18: 235-51. https://doi.org/10.2217/fca-2021-0058 PMid:34841884 |
||||
| 8.Rouhana S, Virsolvy A, Fares N, Richard S, Thireau J. Ranolazine: an old drug with emerging potential; lessons from pre-clinical and clinical investigations for possible repositioning. Pharmaceuticals (Basel) 2021; 15: 31. https://doi.org/10.3390/ph15010031 PMid:35056088 PMCid:PMC8777683 |
||||
| 9.Ghosh GC, Ghosh RK, Bandyopadhyay D, Chattopadhyay A, Banerjee A. Ranolazine: multifaceted role beyond coronary artery disease-a recent perspective. Heart Views 2018; 19: 88-98. https://doi.org/10.4103/HEARTVIEWS.HEARTVIEWS_18_18 PMid:31007857 PMCid:PMC6448470 |
||||
| 10.Rayner-Hartley E, Sedlak T. Ranolazine: a contemporary review. J Am Heart Assoc 2016; 5: e003196. https://doi.org/10.1161/JAHA.116.003196 PMid:26979079 PMCid:PMC4943285 |
||||
| 11.Byrne RA, Rossello X, Coughlan JJ, Barbato E, Berry C, Chieffo A, et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur Heart J 2023; 44: 3720-826. https://doi.org/10.1093/eurheartj/ehad191 PMid:37622654 |
||||
| 12.Virani SS, Newby LK, Arnold SV, Bittner V, Brewer LP, Demeter SH, et al. 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA Guideline for the management of patients with chronic coronary disease: a report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2023; 148: e9-e119. https://doi.org/10.1161/CIR.0000000000001195 |
||||
| 13.Vrints CJM, Andreotti F, Koskinas KC, Amat-Santos IJ, Baumbach A, Capodanno D, et al. 2024 ESC Guidelines for the management of chronic coronary syndromes. Eur Heart J 2024; 45: 3415-537. https://doi.org/10.1093/eurheartj/ehae177 PMid:39210710 |
||||
| 14.Ishii H, Amano T, Matsubara T, Murohara T. Pharmacological prevention of peri- and post-procedural myocardial injury in percutaneous coronary intervention. Curr Cardiol Rev 2008; 4: 223-30. https://doi.org/10.2174/157340308785160598 PMid:19936199 PMCid:PMC2780824 |
||||
| 15.Cocco G. Indicated and off-label use of ranolazine. EJ Cardiol Pract 2013; 11: 18. | ||||
| 16.De Santis GA, De Ferrari T, Parisi F, Fraccaro E, Di Marino M, Pistelli L, et al. Ranolazine unveiled: rediscovering an old solution in a new light. J Clin Med 2024; 13: 4985. https://doi.org/10.3390/jcm13174985 PMid:39274195 PMCid:PMC11396555 |
||||
| 17.Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 2019; 40: 87-165. https://doi.org/10.1093/eurheartj/ehy394 https://doi.org/10.1093/eurheartj/ehy855 |
||||
| 18.Fayed HM, Alsenbesy MA, Saleem TH. Comparative study of circulating cardiac biomarker Galectin-3 and Troponin I in heart failure patients. Clin Med Diagn 2013; 3: 92-100. | ||||
| 19.Elsewify WAE, Ashry MA, Elsaied AA, Abdelrazek EM. Validity of B-type natriuretic peptide, growth differentiation factor 15, and high-sensitivity Troponin I levels in ischemic heart failure. Clin Lab 2022; 68. https://doi.org/10.7754/Clin.Lab.2021.210751 PMid:35536060 |
||||
| 20.Ganesha Babu G, Hausenloy DJ, Yellon DM. Peri-procedural myocardial injury during percutaneous coronary intervention: an important target for cardioprotection. Eur Heart J 2011; 32:23-31. https://doi.org/10.1093/eurheartj/ehq393 PMid:21037252 |
||||
| 21.Nienhuis MB, Ottervanger JP, Bilo HJG, Dikkeschei LD, Zijlstra F. Prognostic value of troponin after elective percutaneous coronary intervention: a meta-analysis. Catheter Cardiovasc Interv 2008; 71: 318-24. https://doi.org/10.1002/ccd.21345 PMid:18288753 |
||||
| 22.Alkhateeb A, Abd Elrady NS, Ali AMS, El-Shaer AO, Abdel Aal SM. Impact of diabetes mellitus on acute and short-term left ventricular longitudinal systolic strain recovery after percutaneous coronary intervention in ischemic hypertensive patients. SVU-Int J Med Sci 2023; 6: 152-9. https://doi.org/10.21608/svuijm.2023.202042.1556 |
||||
| 23.Elsenbsy MA, Sabra AM, Ali AEM, Mohamed WA. Left ventricular dysfunction in prediabetic patients: a review article. SVU-Int J Med Sci 2022; 5: 261-7. | ||||
| 24.Pelliccia F, Pasceri V, Marazzi G, Rosano G, Greco C, Gaudio C. A pilot randomized study of ranolazine for reduction of myocardial damage during elective percutaneous coronary intervention. Am Heart J 2012; 163: 1019-23. https://doi.org/10.1016/j.ahj.2012.03.018 PMid:22709755 |
||||
| 25.Zacharowski K, Blackburn B, Thiemermann C. Ranolazine, a partial fatty acid oxidation inhibitor, reduces myocardial infarct size and cardiac troponin T release in the rat. Eur J Pharmacol 2001; 418: 105-10. https://doi.org/10.1016/S0014-2999(01)00920-7 PMid:11334871 |
||||
| 26.Iqbal SMM, Ahsan S, Jahan K. Effectiveness of ranolazine to prevent myocardial injury during elective percutaneous coronary intervention. Anwer Khan Mod Med Coll J 2019; 10: 43-9. https://doi.org/10.3329/akmmcj.v10i1.43664 |
||||
| 27.Kourtis K, Bourazana A, Xanthopoulos A, Skoularigis J, Triposkiadis F, Papamichalis M, et al. Association between ranolazine, ischemic preconditioning, and cardioprotection in patients undergoing scheduled percutaneous coronary intervention. Medicina 2024; 60: 166. https://doi.org/10.3390/medicina60010166 PMid:38256425 PMCid:PMC10820875 |
||||
| 28.Tariq H, Ahmed S, Ahmed S, Khan MT, Shaikh SA, Shahid I, et al. Efficacy of Nicorandil in preventing myocardial injury and cardiovascular outcomes in patients undergoing percutaneous coronary intervention (PCI): a systematic review and meta-analysis. Cureus 2024; 16: e66938. https://doi.org/10.7759/cureus.66938 |
||||
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

