Vascular surgery strategy to preserve function in lower limbs sarcoma resection.: An oncovascular approach
ORIGINAL RESEARCH ARTICLE
Vascular surgery strategy to preserve function in lower limbs sarcoma resection.: An oncovascular approach
Article Summary
- DOI: 10.24969/hvt.2025.586
- CARDIOVASCULAR DISEASES
- Published: 25/08/2025
- Received: 08/05/2025
- Revised: 02/08/2025
- Accepted: 02/08/2025
- Views: 2062
- Downloads: 1167
- Keywords: Sarcoma, limbs sparing, artery reconstruction, vein reconstruction
Address for Correspondence: Fabio Massimo Oddi, Vascular Surgery Unit, Biomedicine and Prevention Department, University of Rome Tor Vergata, Viale Oxford 81, 00133, Rome, Italy
E-mail: fabio.massimo89@gmail.com Phone: +390620902833
ORCID: 0000-0001-8081-807X
Andrea Ascoli Marchetti, Fabio Massimo Oddi, Lorenzo Di Giulio, Mauro Fresilli, Alessandro Ranucci, Federico Francisco Pennetta, Cataldo Caruso, Martina Battistini, Eugenio Martelli
Vascular Surgery Unit, Biomedicine and Prevention Department, University of Rome Tor Vergata, Rome, Italy
Abstract
Objective: All strategy should be adopted to preserve limb function in case of limbs bone sarcoma. The aim of the study is to share our experience on intervention, results and complications of the performed vascular surgery and reconstructive procedures in patients with lower limb sarcoma.
Methods: From 2011 to 2023, among 32 patients with musculoskeletal neoplasms with a mean age of 44 years (14-67 years), 28 cases (87.5%) underwent revascularization. The study included a biopsy of the lesion, ultrasonography and computed tomography angiography to evaluate of the vascular anatomy, neoplasia extension and postoperative course. The follow-up was 20.1 months.
Results: The reconstruction of the vessels has been implemented in 14/28 (50%) mainly using the autologous saphenous contralateral vein. The use of the prostheses (PTFE and Dacron K) was performed in the other 50% of cases. In five cases (15.6%), the patients had lower limb edema. After vascular surgery, a venous patency restored in 26/32 (80%) and arterial - 100%. No mortality was noted at 30 days. Two patients underwent vacuum assisted closure therapy. In 14 patients perioperative chemotherapy or radiotherapy was performed.
Conclusions: Major vessel involvement during cancer surgery may be considered also in musculoskeletal tumors. The vascular competence in a multidisciplinary approach, plans the exact surgical view through the avascular plane far from neoplasm and may prevent or minimize the bleeding complications, assuring definitive result of the resection.
Key words: Sarcoma, limbs sparing, artery reconstruction, vein reconstruction
Introduction
The vascular management of patients affected by resectable neoplasms provides the use of principles of oncovascular surgery in operative planning, particularly when preparing for a ligation with complete removal or when performing an arterial or venous reconstruction. In patients with lower limb soft tissue sarcomas, the presence of involvement of the neurovascular structures is 3%. Although it is therefore a rare necessity, this type of involvement requires vascular surgery skills that must be considered (1, 2).
The aim of the study is to share our experience on the intervention, results and complications of the performed vascular surgery and reconstructive procedures in patients with lower limb sarcoma.
Graphical abstract
Methods
Study design and population
The study design is a retrospective cohort study. In a period from 2011 to 2022, in our center 32 patients with a mean age of 44 years (14-67 years) affected by primary and secondary musculoskeletal neoplasms (lower limb sarcoma) were treated. In 28 cases (87.5%) revascularization was required.
All subjects gave their informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee (approval number code 19121). Informed consent for the study was obtained from all patients. Written informed consent was obtained from all patients for the procedure and the potential publication of their clinical data.
Data
We analyzed age, sex of patients, frequency of operative factors including type of revascularization, graft details, patency and postoperative complications, indications for surgical revascularization and technique, hospital and 30-day mortality, amputation and re-intervention rates, therapy.
Preoperative evaluation
The preoperative study included a biopsy of the lesion and ultrasonography (USG) and computed tomography angiography (CTA) with multiplanar reconstructions for the evaluation of the vascular anatomy, neoplasia extension and a consequent accurate planning of the intervention after oncological, orthopedic and vascular surgery opinion.
Vascular surgery
The choice of material for revascularization was guided by the following criteria: 1) The length of the graft for the musculoskeletal segment considered: the greater the extent of surgical excision, the greater the use of autologous material; 2) The presence of immunosuppression due to concomitant immunosuppressive therapies. In this case, too, autologous material was preferred to prevent the risk of infection; 3) The presence of both arterial and venous segments to be replaced. Vein harvesting for replacement, already planned for the arterial segment, was also considered for the venous segment.
Allogeneic prosthetic material was chosen for short segments (<10), in cases of arterial-only revascularization, in patients who were not immunosuppressed and had not undergone recent chemotherapy, and with normal blood counts. Furthermore, other parameters personalized for each patient were considered, such as the unavailability of venous material, diabetes, previous interventions or evaluations for the preservation of the venous system in case of further needs.
.
Postoperative evaluation
Postoperative data included; perioperative morbidities such as graft thrombosis bleeding, infection and mortality.
Patients with arterial reconstruction were discharged on 100 mg of acetylsalicylic acid daily. Patients with venous repair underwent a color flow Duplex USG scan during the follow up period and in case of suggestive findings for deep vein thrombosis (DVT). Patients with a DVT received oral anticoagulation with dicumarol or novel oral anticoagulants therapy that continued for 6 months.
The follow up physical examination included ankle-brachial index (ABI) and USG duplex scan in order to assess the patency of the grafts. During the follow-up, postoperative edema was monitored for venous insufficiency of the operated extremity. The patients were followed up in 30 days, sixth month, 1 year and then annually. The success of the arterial reconstruction or venous insufficiency was assessed by clinical examination regularly. ABI by color duplex USG was done, in order to prove the efficacy of reconstruction. The patients were examined postoperatively by an orthopedic surgeon also.
Statistical analysis
IBM SPSS Statistics (IBM Corp., Armonk, New York, NY, USA, Version 20) was used for statistical analysis. Categorical variables are presented as values with percentages.
Results
As can be seen from Table 1, the mean age of patients was 44 years (14–67). Women accounted for 46.9% (15/32), 53.1% were men (17/32).
|
Table 1. Results of vascular reconstruction to preserve function in lower limbs sarcoma resection. |
|
|
Variable |
Data |
|
Number of patients |
32 |
|
Mean age (range), years |
44 (14–67) |
|
Sex (M/F) |
17/15 |
|
Diagnosis |
Musculoskeletal neoplasms (primary/secondary) |
|
Imaging modality |
Biopsy, Ultrasonography, CTA |
|
Type of graft used: |
|
|
- IGVS autologous vein, n(%) |
14 (43.7%) |
|
- PTFE/Dacron, n(%) |
18 (56.3%) |
|
Venous patency, n(%) |
26 (80%) |
|
Arterial patency, n(%) |
100% |
|
Postoperative edema, n(%) |
5 (15.6%) |
|
VAC therapy required, n |
2 |
|
Chemotherapy/radiotherapy, n |
14 |
|
Medium follow-up, months |
20.01 |
|
30-day mortality, % |
0 |
|
30-day amputations, % |
0 |
|
CTA – computed tomography angiography, PTFE – polytetrafluoroethylene, VAC -vacuum assisted closure, IGVS – inverted great saphenous vein |
|
The vascular reconstruction was performed in 28 of 32 patients. The reconstruction of the vessels has been implemented in 14/32 (43.7%) mainly using the autologous saphenous contralateral vein. The use of the prosthesis was performed in the others 56.3% of cases (with PTFE and Dacron K prosthesis). Two patients underwent vacuum assisted closure (VAC) therapy. In one case, transplantation of bone was associated.
In 5 cases (15.6%) the patients had lower limb edema. A distance venous patency restored in 26/32 (80%) and arterial patency - 100%. No mortality at 30 days was found. No amputations were performed at 30 days. The average postoperative follow-up was 20.1 months. In 14 patients perioperative chemotherapy or radiotherapy was performed. Four 4 patients of 14 (28.5%) who underwent autologous vein reconstruction presented with an asymptomatic occlusion of the vein graft. Postoperative edema was treated with compression therapy and was successfully in all cases. An overview of the results is provided in Table 1.
Discussion
The first reported case of vascular reconstruction associated with lover limb sarcoma resection was described by Fortner in 1977 (3). The concept of limbs preserving surgery has been progressively adopted, when possible, for the improvement of the quality of life (4). The oncovascular approach is the evaluation concerning the involvement of vessels in the resection of sarcomas of the limbs. In most cases, when the neoplasm completely surrounds the artery, there is an indication for block resection and replacement of the artery with a autogenous prosthesis, if available (5, 6).
Multidisciplinary approach
The approach with more than one competence should be an objective in all invasive neoplasms with vascular involvement. The decision-making process in extensive lower limbs soft tissue sarcoma, involving vessels, is crucial and include oncologist, orthopedic and vascular specialists for a preoperative planning and cooperation also in the postoperative period (3). Curiously, in the recent guidelines for clinical practice in the treatment of bone sarcomas, there are no criteria indicated for the possible revascularization, even if the advantage in preserving the function is indicated (7, 8).
From oncological point of view, the risk of femur fracture should be calculated during radiotherapy. Folkert et al. (9) estimated 6.7% in their experience, calculating the predictors of femur fracture, such as gender, tumor size, and dose of radiotherapy.
From surgical point of view, there is a need of an adequate preoperative planning and surgical technique, to minimize the risk of an inadequate resection (10). Lower limb soft tissue sarcoma can rarely be removed preserving the arterial and venous vessels because major vascular resection and reconstruction is required for adequate oncologic margins. The excision with artery and/or vein reconstruction has been already reported. As example, we report a case of male patient 41 years old, affected by symptomatic extraskeletal Ewing sarcoma with vascular compression, which is included in this series. An “in block resection” with bone transplantation and vessel replacement with saphenous venous graft was performed (Fig. 1).
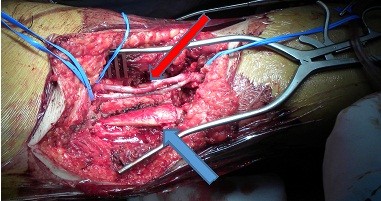
Figure 1. Intraoperative image. Prone patient. Posterior view. The sarcoma was removed. The arrow indicates the autologous reversed saphenous vein graft of the superficial femoral artery and vein (red arrow) with the bone transplanted, after femor excision (blue arrow)
Preoperative diagnostic imaging
Preoperative vascular imaging is crucial for operative planning. In our series, all patients underwent CTA. This examination give, using multiplanar reconstruction, the best imaging requirements for preoperative evaluation in tumor resection surgery, accurately revealing the vascularization of the neoplasm and the relationships of contiguity or continuity between the neoplasm and the vessels (Fig. 2A). CT gives best depiction of osseous structures and has better spatial resolution compared to magnetic resonance imaging, despite the disadvantages of using ionizing radiation and iodine contrast medium, potentially nephrotoxic. In all cases, patients had the contralateral leg vein mapped just before the operation to obtain precise donor site of the great saphenous vein, in case of need.
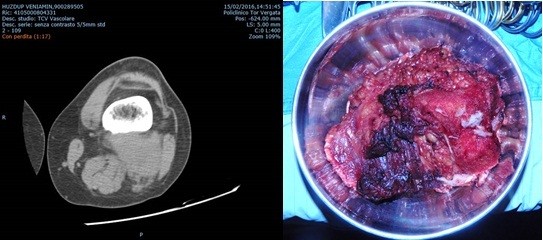
A) B)
Figure 2. A preoperative computed tomography scan: extraskeletal Ewing sarcoma. Note the bone erosion and vessel compression. B) Intraoperative specimen of neoplasm after the surgical removal
Perioperative course
The surgical techniques in previous series reports mortality rates of 0-4.8%, tumor control in 86-100% of patients and limb salvage in 92-94.1% (3). In the postoperative period, the presence of edema was clinically evident in about 40% of treated cases, with no obvious difference between veins treated with grafting or with ligation as Schwarzbach, Tsukushi and Spark demonstrated (1, 4, 11). In our experience 5 of 32 patients presented edema postoperative with the same characteristics. Morbidity came from wound complications, with dehiscence reported in the literature in 33-57% of cases (12). In our cases, this kind of complication were successfully treated with VAC. The literature reports a graft infection rates of 6-29%. (5, 13, 14, 16) (Table 2). No major infection were observed in our experience. In this condition, when the vessel involvement and removal is crucial for a radical surgery, vascular surgeon role is essential in a multidisciplinary setting, also to choice the better technique of revascularization or to decide no indication for an eventual venous vessel replacement (15–18) (Fig. 3, 4).
In particular cases the removal of the neoplasm may require a large dissection to preserve the vascular axis and also in the absence of a revascularization, the presence of the vascular surgeon makes the bleeding minimal and the removal more accurate and, at the same time, radical (Fig. 2B). While immediate results are influenced by intraoperative conduct, the follow-up results are conditioned by the appearance of metastases and consequent mortality is not negligible. The 5-year survival rate is 68.4% in the more prolonged follow up (17,18). As in reconstructions for atherosclerotic obstructive pathology, the vein is used as an ideal substitute. In our experience, in the case of unavailability of suitable venous autogenic substitutes, good results have also been obtained with synthetic prostheses with comparable rates of patency. It is necessary to consider that these are normal arteries, therefore with greater possibility of patency even at a distance. For venous reconstructions also in our experience the patency was lower, but with no relevant symptoms. The majority of patients must perform chemotherapy and radiotherapy cycles and despite the good results, the mortality at distance is unpredictable, depending on the neoplasm prognosis.
|
Table 2. Literature review of outcomes of vascular reconstructions in musculoskeletal tumors of the limbs |
|||||||
|
Author |
Period |
Pts, n |
Mean follow -up, months |
Artery involvement and % of artery reconstrution |
30- day primary patency, % |
Local infection, n (%) |
Mortality 30-day, % |
|
Karakousis (19)
|
1991-1996 |
21 |
60 |
7/21 33.0 |
100 |
5 (24.0) |
0 |
|
McKay (12)
|
2002-2005 |
7 |
20.2 |
7/7 100 |
100 |
0 |
0 |
|
Spark (4)
|
2002-2006 |
9 |
19.1 |
8/9 88.8 |
100 |
NR |
0 |
|
Akgul T (16)
|
2004-2007 |
15 |
39 |
12/15 80 |
82.4 |
5 (29.4) |
- |
|
Song (20)
|
2003-2008 |
14 |
36 |
7/14 50.0 |
83 |
0 |
0 |
|
Muramatsu (14)
|
1995-2010 |
15 |
71.8 |
15/15 100 |
93.3 |
1 (6) |
0 |
|
Davis (15)
|
2005-2013 |
9 |
74.7 |
1/9 11.1 |
NR |
NR |
0 |
|
Cetinkaya (18)
|
2002-2014 |
13 |
80.6 |
13/13 100 |
84.6 |
3 (23.1) |
0 |
|
Our experience |
2011-2022 |
32 |
20.1 |
14/32 43.7 |
100 |
2 (6.6) |
0 |
|
NR – not reported, pts - patients |
|||||||
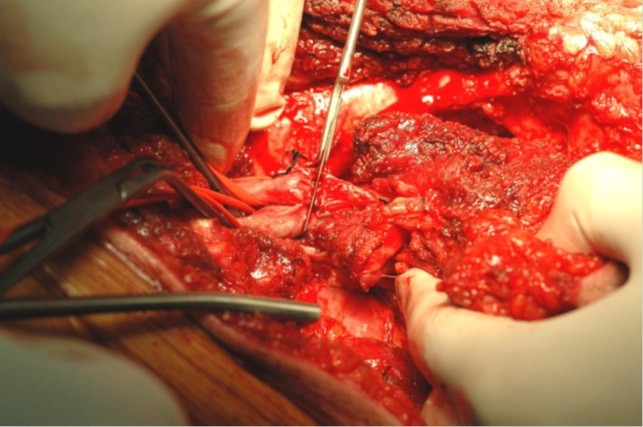
Figure 3. Intraoperative image. The scalpel resets the neoplasm proximally. The noninvolved vessels are clamped. The arrow indicates the superficial femoral artery and vein to the left of the scalpel (Republished from ref. 21 under CC BY license 4.0, authors collection)
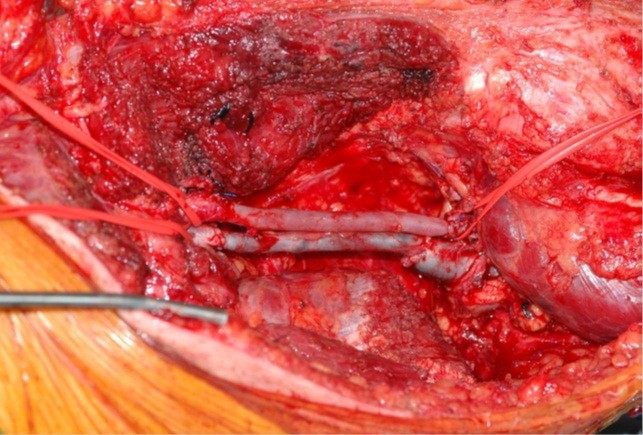
Figure 4. Intraoperative image. The superficial femoral artery and the superficial femoral vein are removed with the sarcoma. An IGVS (inverted great saphenous vein) has been grafted to replace the removed artery tract. The great saphenous vein has been grafted to replace the removed vein tract (Republished from ref. 21 under CC BY license 4.0, authors collection)
Study limitations
The main limitation of this study lies in its retrospective, single-center design and the relatively small sample size, which may limit the generalizability of the findings and preclude robust statistical comparisons across subgroups.
Conclusion
Major vessel involvement during cancer surgery may be considered also in musculoskeletal tumors. The vascular surgeons or competence in a multidisciplinary approach, plans the exact surgical view through the avascular plane far from neoplasm and may prevent or minimize the bleeding complications, assuring definitive result of the resection. A long-term evaluation is needed to determine the implications at distance in these patients. The necessity of vascular surgery skills is rare but essential for limb sparing. The mortality gap is conditioned by the prognosis of neoplasm.
Ethics: All subjects gave their informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee (approval number cod. 19121). Informed consent for the study was obtained from all patients. Study data and materials have been collected and are available on request.
Peer-review: External and internal
Conflict of interest: The authors declare that they have no competing interests
Authorship: A.A.M. participated in the conception and design, operations, analysis and interpretation and drafting the manuscript, critical revision and final approval; F.M.O. participated in the design, operations, analysis and interpretation, drafting the manuscript, revision and final approval; L.D.G. participated in design, operations, drafted the manuscript and final approval; M.F., A.R., F.F.P., C.C., and M.B. participated in the data collection and analysis and final approval; M.E. participated in the conception and design, operations, analysis and interpretation and drafting the manuscript, critical revision and final approval. Thus all authors fulfilled authorship criteria.
Acknowledgements: The authors would like to acknowledge the teams of Oncological Orthopedics of IFO (R. Biagini and colleagues), Orthopedic and Traumatology A and B Unit of the Tor Vergata University (E. Ippolito, U. Tarantino and P. Farsetti and colleagues) and the former members of Vascular Surgery Unit of Tor Vergata University (A. Ippoliti, R. Ciattaglia, G. Citoni) for their collaboration.
Funding: The authors declare no funds have been paid for research, for body in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Statement on A.I.-assisted technologies use: We declare that we did not use AI-assisted technologies in preparation of this manuscript
Availability of data and materials: The data supporting their findings can be found in the electronic archive of our hospital and are available on request.
References
| 1. Schwarzbach MH, Hormann Y, Hinz U, Bernd L, Willeke F, Mechtersheimer G, et al. Results of limb-sparing surgery with vascular replacement for soft tissue sarcoma in the lower extremity. J Vasc Surg 2005; 42: 88-97. https://doi.org/10.1016/j.jvs.2005.03.017 PMid:16012457 |
||||
| 2.Tunn PU, Kettelhack C, Dürr HR. Standardized approach to the treatment of adult soft tissue sarcoma of the extremities. Recent Results Cancer Res 2009;179: 211-28. https://doi.org/10.1007/978-3-540-77960-5_13 PMid:19230542 |
||||
| 3. Fortner JG, Kim DK, Shiu MH. Limb-preserving vascular surgery for malignant tumors of the lower extremity. Arch Surg 1977; 112: 391-4. https://doi.org/10.1001/archsurg.1977.01370040043007 PMid:849146 |
||||
| 4.Spark JI, Charalabidis P, Laws P, Seben R, Clayer M. Vascular reconstruction in lower limb musculoskeletal tumours. ANZ J Surg 2009; 79: 619-23. https://doi.org/10.1111/j.1445-2197.2009.05016.x PMid:19895517 |
||||
| 5. Ghosh J, Bhowmick A, Baguneid M. Oncovascular surgery. Eur J Surg Oncol 2011; 37: 1017-24. https://doi.org/10.1016/j.ejso.2011.08.131 PMid:21917411 |
||||
| 6. Han SM. Oncovascular Surgery: There would be no such thing without vascular surgeons. Vasc Specialist Int 2019; 35: 53-4. https://doi.org/10.5758/vsi.2019.35.2.53 PMid:31297353 PMCid:PMC6609018 |
||||
| 7. Casali PG, Bielack S, Abecassis N, Aro HT, Bauer S, Biagini R, et al; ESMO Guidelines Committee, PaedCan and ERN EURACAN. Bone sarcomas: ESMO-PaedCan-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018; 29(Suppl 4): iv79-iv95. | ||||
| 8. Nishinari K, Wolosker N, Yazbek G, Zerati AE, Nishimoto IN. Venous reconstructions in lower limbs associated with resection of malignancies. J Vasc Surg 2006; 44: 1046-50. https://doi.org/10.1016/j.jvs.2006.07.033 PMid:17098540 |
||||
| 9. Folkert MR, Casey DL, Berry SL, Crago A, Fabbri N, Singer S, et al. Femoral fracture in primary soft-tissue sarcoma of the thigh and groin treated with intensity-modulated radiation therapy : Observed versus expected risk. Ann Surg Oncol 2019; 26: 1326-31. https://doi.org/10.1245/s10434-019-07182-5 PMid:30706225 PMCid:PMC6458075 |
||||
| 10. Biau DJ, Weiss KR, Bhumbra RS, Tr F, Davidson D, Brown C, et al. Monitoring the adequacy of surgical margins after resection of bone and soft-tissue sarcoma. Ann Surg Oncol 2013; 20: 1858-64. https://doi.org/10.1245/s10434-012-2863-8 PMid:23370669 |
||||
| 11. Tsukushi S, Nishida Y, Sugiura H. Results of limb-salvage surgery with vascular reconstruction for soft tissue sarcoma in the lower extremity: Comparison between only arterial and arterovenous reconstruction. J Surg Oncol 2008; 97: 216-20. https://doi.org/10.1002/jso.20945 PMid:18161869 |
||||
| 12.McKay A, Motamedi M, Hons BA, Temple W. Vascular reconstruction with the superficial femoral vein following major oncologic resection. J Surg Oncol 2007; 96: 151-9. https://doi.org/10.1002/jso.20788 PMid:17443742 |
||||
| 13. Adelani MA, Holt GE, Dittus RS, Passman MA. Revascularization after segmental resection of lower extremity soft tissue sarcomas. J Surg Oncol 2007; 95: 455-60. https://doi.org/10.1002/jso.20679 PMid:17458861 |
||||
| 14. Muramatsu K, Ihara K, Miyoshi T, Yoshida K, Taguchi T. Clinical outcome of limb-salvage surgery after wide resection of sarcoma and femoral vessel reconstruction. Ann Vasc Surg 2011; 25: 1070-7. https://doi.org/10.1016/j.avsg.2011.05.009 PMid:21831587 |
||||
| 15. Davis LA, Dandachli F, Turcotte R, Steinmetz OK. Limb-sparing surgery with vascular reconstruction for malignant lower extremity soft tissue sarcoma. J Vasc Surg 2017; 65: 151-6. https://doi.org/10.1016/j.jvs.2016.05.094 PMid:27687325 |
||||
| 16. Akgül T, Sormaz C, Aksoy M, Ozger H, Eralp L. Results and functional outcomes of en-bloc resection and vascular reconstruction in extremity musculoskeletal tumors. Acta Orthop Traumatol Turc 2018; 52: 409-14. https://doi.org/10.1016/j.aott.2018.08.004 PMid:30274704 PMCid:PMC6318543 |
||||
| 17. Radaelli S, Fiore M, Colombo C, Ford S, Palassini E, San R, et al. Vascular resection en-bloc with tumor removal and graft reconstruction is safe and effective in soft tissue sarcoma ( STS ) of the extremities and retroperitoneum. Surg Oncol 2016; 25: 125-31. https://doi.org/10.1016/j.suronc.2016.05.002 PMid:27566012 |
||||
| 18. Cetinkaya OA, Celik SU, Kalem M, Basarir K, Koksoy C, Yildiz HY. Clinical characteristics and surgical outcomes of limb-sparing surgery with vascular reconstruction for soft tissue sarcomas. Ann Vasc Surg 2019; 56: 73-80. https://doi.org/10.1016/j.avsg.2018.09.018 PMid:30500640 |
||||
| 19. Karakousis CP. Refinements of surgical technique in soft tissue sarcomas. J Surg Oncol 2010; 101: 730-8. https://doi.org/10.1002/jso.21572 https://doi.org/10.1002/jso.21563 PMid:20512950 |
||||
| 20. Song TK, Harris Jr EJ, Raghavan S. Major blood vessel reconstruction during sarcoma surgery. Arch Surg 2009; 144: 817-22. https://doi.org/10.1001/archsurg.2009.149 PMid:19797105 |
||||
| 21. Ascoli Marchetti A, Di Giulio L, Citoni G, Ippoliti A. Oncovascular surgery-The multidisciplinary approach: surgical resection of the musculoskeletal tumor and associated revascularization. J Surg Res 2019: 2; 147-53. https://doi.org/10.26502/jsr.10020031 |
||||
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

