Unloading strategies in venoarterial extracorporeal membrane oxygenation: Insights from a contemporary cohort
ORIGINAL RESEARCH ARTICLE
Unloading strategies in venoarterial extracorporeal membrane oxygenation: Insights from a contemporary cohort
Article Summary
- DOI: 10.24969/hvt.2025.593
- CARDIOVASCULAR DISEASES
- Published: 10/09/2025
- Received: 22/07/2025
- Revised: 26/08/2025
- Accepted: 27/08/2025
- Views: 2044
- Downloads: 1329
- Keywords: Cardiogenic shock; intra-aortic balloon pump; microaxial flow pump; unloading; venoarterial extracorporeal membrane oxygenation
Address for Correspondence: Marta Ludovina Loureiro Fernandes Leite, Rua Conceição Fernandes, Unidade Local de Saúde de Gaia e Espinho, 4430-000, Vila Nova de Gaia, Portugal
E-mail: martaleite95@gmail.com Phone: +35 1918188720
ORCID: Marta Leite - 0000-0003-4067-3069
Marta Leite1, Fábio Sousa-Nunes1,2, Mariana Brandão1, Mariana Ribeiro Silva1, Pedro Gonçalves Teixeira1, Marisa Silva1, Marta Ponte1, Gustavo Pires-Morais1, Adelaide Dias1, Pedro Braga1, Daniel Caeiro1, Ricardo Fontes-Carvalho1,2
1Cardiology Department, Unidade Local de Saúde Gaia/Espinho, 4434-502 Vila Nova de Gaia, Portugal
2Cardiovascular R&D Centre - UnIC@RISE, Faculty of Medicine of the University of Porto, 4200-319 Porto, Portugal
Abstract
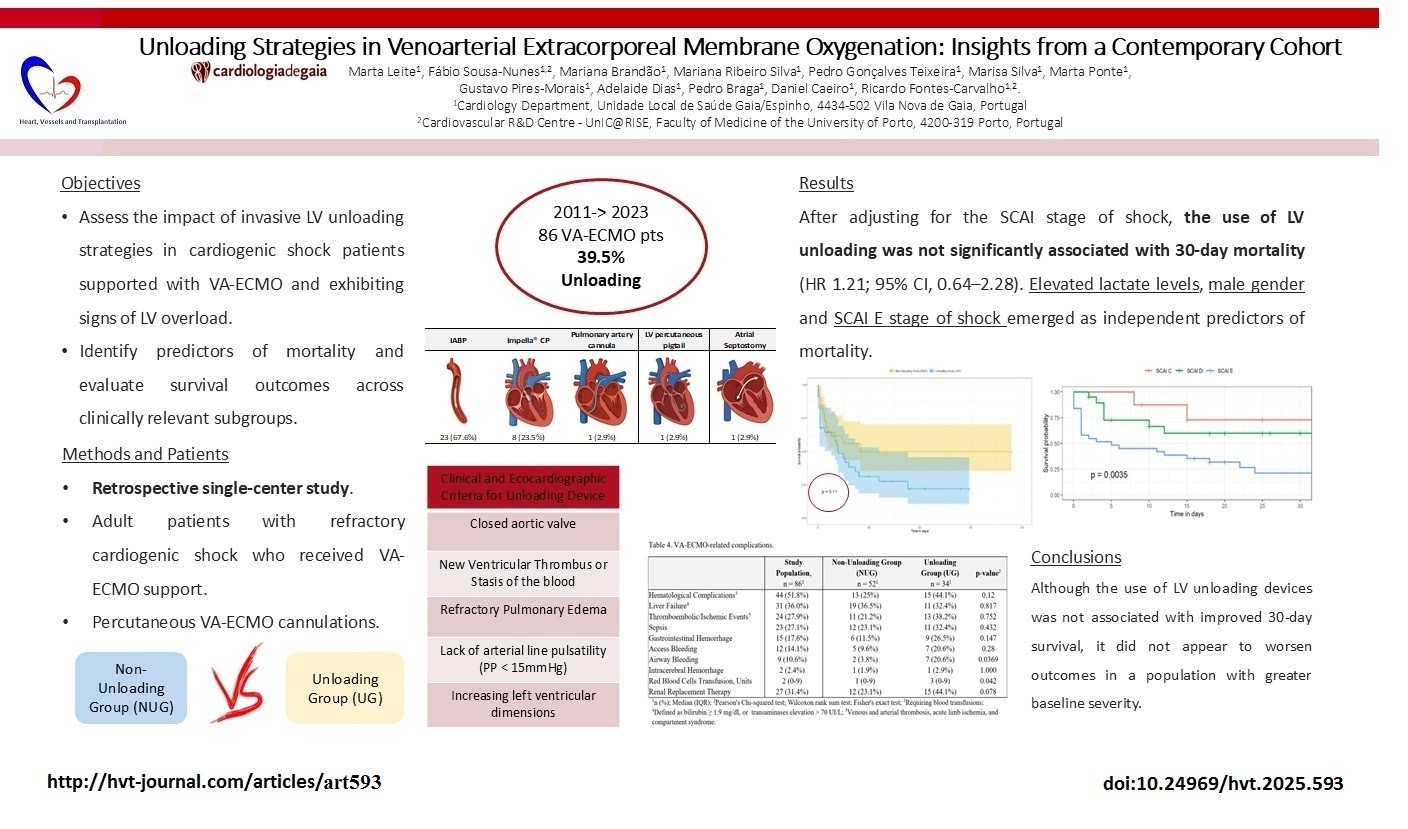
Objective: Venoarterial extracorporeal membrane oxygenation (VA-ECMO) provides temporary support in refractory cardiogenic shock but can exacerbate left ventricular (LV) overload. LV unloading strategies, such as intra-aortic balloon pump (IABP) and microaxial flow pumps (e.g., Impella®), have been proposed to mitigate these effects, but their survival benefit remains uncertain. This study aimed to assess the impact of invasive LV unloading strategies in cardiogenic shock patients supported with VA-ECMO and signs of LV overload.
Methods: We conducted a retrospective study of patients with refractory cardiogenic shock treated with VA-ECMO at a single tertiary center. The primary endpoint was 30-day survival. Outcomes were compared between patients who underwent invasive LV unloading and those managed without unloading, using logistic regression and Kaplan–Meier analysis.
Results: A total of 86 patients were included [mean age 54.5 (11.8) years; 62% male] and LV unloading was performed in 34 patients (39.5%). The overall 30-day survival rate was 41% (95% CI, 30–55%), with no significant difference between the unloading group (31%, 95% CI 19-53%) and the non-unloading group (50%, 95% CI 35-70%) (p = 0.11). LV unloading was associated with a hazard ratio for 30-day mortality of 1.64 (95% CI, 0.30–1.68), and 1.21 (95% CI, 0.64–2.28) after adjustment for SCAI stage of shock. Predictors of mortality included higher baseline lactate (HR 1.09, 95% CI, 1.00–1.19), male gender (HR 2.99, 95% CI, 1.29–6.98) and SCAI E (HR 2.24; 95% CI, 1.12–4.47). Airway bleeding was more frequent in the unloading group (20.6% vs. 3.8%; p = 0.0369).
Conclusion: LV unloading was not associated with improved 30-day survival in VA-ECMO-treated patients with refractory cardiogenic shock, but may offer potential benefit in selected high-risk patients.
Key words: Cardiogenic shock; intra-aortic balloon pump; microaxial flow pump; unloading; venoarterial extracorporeal membrane oxygenation.
![]()
Introduction
Despite considerable scientific advances in the field of cardiac intensive care over the past two decades, as well as the widespread use of early coronary revascularization in acute myocardial infarction, the mortality rate for cardiogenic shock remains alarmingly high (1–3). For years, mechanical circulatory support (MCS), particularly venoarterial extracorporeal membrane oxygenation (VA-ECMO), has been considered a therapeutic option to improve outcomes in this population (4–6).
VA-ECMO is a salvage therapy for patients with refractory cardiogenic shock, serving as a bridge to decision, recovery, transplantation, or long-term MCS. It can be implanted percutaneously or surgically in different configurations, providing temporary circulatory and respiratory support to critically ill patients (7). Despite its growing use, patients with cardiogenic shock supported with VA-ECMO continue to exhibit high mortality rates, with early mortality ranging from 40% to 75% (8–10).
A critical challenge associated with VA-ECMO therapy is the non-physiological high flow delivered by the peripheral arterial cannula against a severely dysfunctional left ventricle (LV), increasing LV afterload and reducing stroke volume. As the LV attempts to overcome the retrograde flow, wall tension and end-diastolic pressure rise, contributing to blood stasis, thrombosis, and pulmonary edema (11). Daily management of VA-ECMO patients reveals a pathological increase in afterload with significant hemodynamic consequences in more than half of the patients (11).
LV unloading in VA-ECMO patients may promote myocardial recovery by lowering oxygen consumption and mechanical stress on the heart. Conservative measures may initially be implemented with inotropic or diuretic agents. More invasive strategies include the percutaneous placement of a LV pigtail, a venting cannula on the pulmonary artery, atrial septostomy, or the use of percutaneous LV assist devices, such as intra-aortic balloon pump (IABP) and transvalvular microaxial flow pumps, such as the Impella® device (12–14). Nevertheless, the survival benefits of unloading strategies remain unproven, and the addition of other MCS devices may increase the risk of bleeding and thrombotic complications (14).
This study aimed to assess the impact of invasive LV unloading strategies in cardiogenic shock patients supported with VA-ECMO and exhibiting signs of LV overload. Additionally, we sought to identify predictors of mortality within this cohort and to evaluate survival outcomes across clinically relevant subgroups.
Methods
Study design and population
We conducted a retrospective, single-center study including adult patients (≥18 years) with refractory cardiogenic shock who received VA-ECMO support at a tertiary care center between January 2011 and December 2023. Patients were identified through institutional databases and ECMO logs. Data extraction and analysis were performed between January and August 2024. Inclusion criteria comprised a diagnosis of refractory cardiogenic shock or cardiac arrest requiring VA-ECMO. Exclusion criteria included post-cardiotomy cardiogenic shock or insufficient clinical data for analysis. The first forty-eight cases were previously published in Revista Portuguesa de Cardiologia in 2017 by Passos Silva et al. (15), and we followed a similar study design.
Patients were divided into two groups depending on whether an LV unloading intervention was performed: the Unloading Group (UG) and the Non-Unloading Group (NUG).
This study was conducted in accordance with the Declaration of Helsinki. Given the emergent and life-threatening condition of the patients included in this study, when informed consent for procedures could not be obtained prior to initiation, treatment was provided in accordance with standard emergency protocols.
Data
Baseline patient characteristics, primary diagnoses, shock severity, and treatment strategies were collected. The severity of cardiogenic shock was assessed using hemodynamic parameters, arterial lactate levels, and validated scoring systems, namely the Survival After Veno-arterial ECMO (SAVE) score and the Society for Cardiovascular Angiography and Interventions (SCAI) shock stage classification (16,17). Vital signs were recorded immediately before VA-ECMO implantation, excluding cases of extracorporeal cardiopulmonary resuscitation (E-CPR). The same approach was applied to laboratory values, which were obtained prior to VA-ECMO initiation or, if unavailable, the first recorded values following cannulation were used.
VA-ECMO and unloading strategies
All VA-ECMO cannulations in our cohort were performed percutaneously. The decision to initiate LV unloading, including the choice and timing of the device, was based on individual clinical assessment by the treating team, taking into account the patient's hemodynamic status, left ventricular loading conditions, and device availability.
Clinical and echocardiographic criteria that prompted the implementation of unloading devices were based on current literature and included: LV dilatation; reduced aortic valve opening as evidenced by diminished arterial line pulsatility or echocardiographic imaging; pulmonary edema detected by lung ultrasound, chest X-ray, or hypoxemia; and direct measurement of pulmonary artery pressure using a pulmonary artery catheter (12, 13).
Techniques employed included IABP, microaxial flow pumps (Impella®), atrial septostomy, and other percutaneous decompression approaches such as LV pigtail insertion or pulmonary artery drainage. LV unloading strategies were stratified as early (within the first 24-hours of VA-ECMO initiation) or late (after 24-hours) during the course of support.
Endpoints
The primary endpoint of this study was the 30-day survival rate, comparing patients who underwent invasive unloading strategies with those who did not. Events occurring beyond 30 days were censored.
Secondary endpoints included the length of hospitalization and the duration of VA-ECMO support. Additionally, baseline predictors of 30-day mortality were evaluated, along with the potential independent association between LV unloading and mortality.
Safety endpoints encompassed VA-ECMO-related complications, including significant clinical bleeding events, vascular complications associated with peripheral access, and ischemic events. Moderate to severe bleeding complications were reported according to GUSTO (Global Use of Strategies to Open Coronary Arteries) criteria (18). Hematologic complications were reported when patients required blood transfusions due to hematologic dysfunction. Liver failure was defined as a serum bilirubin level ≥ 1.9 mg/dL, accompanied by elevations in alanine transaminase (ALT) and/or aspartate transaminase (AST) superior to 70 UI/L, according to laboratory cut-off values. Thromboembolic and ischemic events included ischemic stroke, venous and arterial thrombosis, acute limb ischemia, and compartment syndrome. Sepsis was defined as a confirmed infection causing organ dysfunction. The need for renal replacement therapy was also assessed.
Statistical analysis
Statistical analyses were performed using R statistical software (version 4.3.1; R Foundation for Statistical Computing, Vienna, Austria) and RStudio (version 2023.06.1, Build 524; RStudio, PBC, Boston, MA).
Categorical variables are presented as numbers and percentages, and group comparisons were performed using Pearson’s Chi-squared test, which assesses whether observed differences in categorical distributions are statistically significant.
When expected frequencies in any cell were <5, we used the Fisher’s exact test, which is more appropriate for small sample sizes.
Continuous variables are reported as both means with standard deviations (SD) and medians with interquartile ranges (IQR), as appropriate. To compare continuous variables we used the Wilcoxon rank-sum test, a non-parametric alternative to the t-test that does not assume normal distribution of the data.
Patients were categorized into two groups depending on whether an LV unloading intervention was performed: the Unloading Group (UG) and the Non-Unloading Group (NUG). For survival analysis, Kaplan–Meier curves were generated to estimate 30-day survival, and group comparisons (UG vs. NUG, as well as across SCAI shock stages C, D, and E) were performed using the log-rank test, which assesses differences in survival distributions over time. To evaluate predictors of 30-day mortality, Cox proportional hazards regression was used. This method estimates the hazard (risk) of death associated with various covariates while accounting for time-to-event data. Both univariable and multivariable models were applied. An additional multivariable Cox regression model was performed to evaluate the independent association between LV unloading and 30-day mortality, adjusting for the SCAI stage of cardiogenic shock.
All probability values were two-tailed with p value <0.05, and confidence intervals (CI) were calculated to the 95th percentile.
Results
Study Population
This study included a total of 86 patients. An unloading strategy was employed in 34 patients (39.5%), while no active unloading was performed in 52 patients (60.5%). Baseline patient characteristics are summarized in Table 1.
The mean age of the study population was 54.5 (11.8) years and 62% of the patients were male. Acute coronary syndrome was the most common cause of cardiogenic shock (46.5%), followed by chronic decompensated heart failure (13.9%), electrical storm (10.5%), and myocarditis (8.1%) (Table 1).
When comparing patients receiving VA-ECMO and unloading devices (UG) to those managed without unloading strategies (NUG), both groups were similar regarding gender, age, and cardiovascular comorbidities. However, the primary cause of cardiogenic shock differed between groups: patients in the UG more frequently presented with acute coronary syndrome [16 patients (30.8%) NUG vs. 24 patients (70.6%) UG] or myocarditis [1 patient (1.9%) NUG vs. 5 patients (14.7%) UG], whereas cardiogenic shock in the NUG was more often attributed to chronic decompensated heart failure [10 patients (19.2%) NUG vs. 2 patients (5.9%) UG] or electrical storm [8 patients (15.4%) NUG vs. 1 patient (2.9%) UG] (p=0.003) (Table 1).
Most patients presented with severe, refractory cardiogenic shock at the time of VA-ECMO initiation: mean SAVE score was -9.19 (4.39) and mean lactate levels were 11.0 (5.77) mmol/L. Based on the SCAI shock classification, 54.6% of patients were classified as stage E, 34.9% were stage D, and 10.5% were stage C. There was a trend toward a higher proportion of SCAI stage E patients in the UG [23 patients (44.2%)], whereas SCAI stage D predominated in the NUG [25 patients (48.1%)] (p = 0.055). Patients in the UG presented worse analytical and hemodynamic profiles before VA-ECMO implantation: mean bicarbonate levels were 18.0 (5.00) mmol/L in the NUG compared to 13.7 (5.25) mmol/L in the UG (p = 0.01); mean lactate levels were 9.92 (5.88) mmol/L in the NUG compared to 12.5 (5.58) mmol/L in the UG (p = 0.315); and mean creatinine levels were 1.61 (0.806) mg/dL in the NUG vs. 1.73 (0.739) mg/dL in the UG (Table 1).
More than half of the patients (54.6%) experienced cardiac arrest prior to VA-ECMO implantation. This percentage is similar to the proportion of patients classified as SCAI stage E, although the two groups were not identical. The mean duration of cardiac arrest until return of spontaneous circulation was 36.1 (29.0) minutes overall — 33.7 (31.6) minutes in the NUG vs. 38.3 (27.0) minutes in the UG (p=0.629). E-CPR was performed in 33.7% of the patients — 15 patients (28.8%) in the NUG vs. 14 patients (41.2%) in the UG (p=0.342) — all of them in a hospital setting (Table 1).![]()
|
Table 1. Baseline patient characteristics |
||||
|
Variables |
Study population (n = 861) |
Non-Unloading Group (n = 521) |
Unloading Group (n = 341) |
p2 |
|
Gender, n(%) |
|
|||
|
Male |
53 (62) |
32 (61) |
21 (62) |
1.000 |
|
Female |
33 (38) |
20 (39) |
13 (38) |
|
|
Age, years |
54.5 (11.8) |
54.9 (12.4) |
54.1 (11.1) |
0.751 |
|
Cardiovascular risk factors, n(%) |
|
|||
|
Dyslipidemia |
42 (48.8) |
27 (51.9) |
16 (47.1) |
0.891 |
|
Arterial hypertension |
36 (41.9) |
22 (42.3) |
15 (44.1) |
1.000 |
|
Diabetes mellitus |
24 (27.9) |
18 (34.6) |
7 (20.6) |
0.367 |
|
Active smoker |
38 (44.2) |
23 (44.2) |
15 (44.1) |
1.000 |
|
History of coronary disease |
13 (15.1) |
12 (23.1) |
2 (5.9) |
0.199 |
|
Heart failure |
12 (13.9) |
8 (15.4) |
2 (5.9) |
0.323 |
|
Diagnosis, n(%) |
|
|||
|
Acute coronary syndrome |
40 (46.5) |
16 (30.8) |
24 (70.6) |
0.003 |
|
Chronic decompensated HF |
12 (13.9) |
10 (19.2) |
2 (5.9) |
|
|
Electrical storm |
9 (10.5) |
8 (15.4) |
1 (2.9) |
|
|
Myocarditis |
6 (8.1) |
1 (1.9) |
5 (14.7) |
|
|
Others3 |
19 (22.1) |
17 (32.7) |
2 (5.9) |
|
|
Cardiac arrest before ECMO, n(%) |
47 (54.6) |
27 (51.9) |
20 (58.8) |
0.684 |
|
E-CPR, n(%) |
29 (33.7) |
15 (28.8) |
14 (41.2) |
0.342 |
|
Time do ECLS, min |
36.1 (29.0) |
33.7 (31.6) |
38.3 (27.0) |
0.629 |
|
Invasive mechanical ventilation, n(%) |
79 (91.9) |
46 (88.5) |
33 (97.1) |
0.236 |
|
Hemodynamic status4 |
|
|
|
|
|
Heart rate, bpm |
104 (37.3) |
109 (43.3) |
96 (23.3) |
0.265 |
|
Mean arterial pressure, mmHg |
55.7 (16.5) |
60 (14.4) |
69 (18.1) |
0.08 |
|
Laboratory values5 |
|
|
|
|
|
AST, U/L |
370 (21-3840) |
138 (21-3840) |
747 (34.0-2100) |
0.564 |
|
ALT, U/L |
223 (19-2140) |
134 (19-2140) |
279 (32.0-1800) |
0.973 |
|
Total bilirubin, mg/dL |
0.45 (0.15-4.91) |
0.46 (0.15-4.91) |
0.45 (0.23-1.55) |
0.179 |
|
Creatinine, mg/dL |
1.67 (0.765) |
1.61 (0.806) |
1.73 (0.739) |
0.63 |
|
BUN, mg/dL |
70.7 (41.4) |
72.8 (48.8) |
68.4 (32.5) |
0.69 |
|
pH |
7.16 (0.201) |
7.22 (0.159) |
7.24 (0.196) |
0.782 |
|
Bicarbonate, mmol/L |
15.8 (5.51) |
18.0 (5.00) |
13.7 (5.25) |
0.01 |
|
Lactate, mmol/L |
11.0 (5.77) |
9.92 (5.88) |
12.5 (5.58) |
0.315 |
|
SAVE score |
-9.19 (4.39) |
-8.26 (4.28) |
-10.2 (4.35) |
0.088 |
|
SCAI shock stage, n(%) |
|
|
|
|
|
C |
9 (10.5) |
4 (7.7) |
4 (11.8) |
0.055 |
|
D |
30 (34.9) |
25 (48.1) |
7 (20.6) |
|
|
E |
47 (54.6) |
23 (44.2) |
23 (67.6) |
|
|
1n (%); Mean (SD); Median (IQR). 2Pearson's Chi-squared test; Wilcoxon rank sum test; Fisher's exact test. 3Others including septic shock, Takotsubo cardiomyopathy, pulmonary embolism, acute valvular disease, aortic dissection or unknown cause. 4Values registered before VA-ECMO implantation, excluding E-CPR cases. 5Values obtained before VA-ECMO implantation or the first value registered. ALT - alanine transaminase, AST - aspartate transaminase, BUN - blood urea nitrogen, bpm – beats per minute, ECLS - extracorporeal life support, VA-ECMO – veno-arterial extracorporeal membrane oxygenation, E-CPR - extracorporeal cardiopulmonary resuscitation, min - minutes, HF – heart, failure, SAVE - Survival After VA- ECMO Score, SCAI - Society for Cardiovascular Angiography and Interventions |
||||
Invasive unloading strategies included IABP counterpulsation in 67.6% of cases, followed by Impella® device in 23.5%, and other techniques - such as atrial septostomy, percutaneous LV pigtail insertion, and pulmonary artery cannulation - in 8.8% of patients (Table 2). In 76.5% of cases, the unloading device was implanted within the first 24 hours of VA-ECMO cannulation, most often simultaneously (61.8%). In a smaller proportion (20.6%), the unloading device was implanted beforehand, with VA-ECMO subsequently initiated due to persistent shock. The mean duration of unloading device was 3 (1-6) days.
|
Table 2. Unloading strategies |
|
|---|---|
|
23 (67.6) |
|
|
Impella® CP, n (%) |
8 (23.5) |
|
Pulmonary artery cannula, n (%) |
1 (2.9) |
|
LV percutaneous pigtail, n (%) |
1 (2.9) |
|
Atrial septostomy, n (%) |
1 (2.9) |
|
IABP- intra-aortic balloon pump, LV - left ventricle |
|
Primary and secondary endpoints
The overall 30-day survival rate in our cohort was 41% (95% CI, 30-55%) (Fig.1). In the UG, the survival rate
was 31% (95% CI, 19-53%), while in the NUG, it was 50% (95% CI, 35-70%) (p=0.11) (Fig. 2). The hazard ratio (HR) for mortality associated with LV unloading was 1.64 (95% CI 0.30–1.68, p = 0.09).
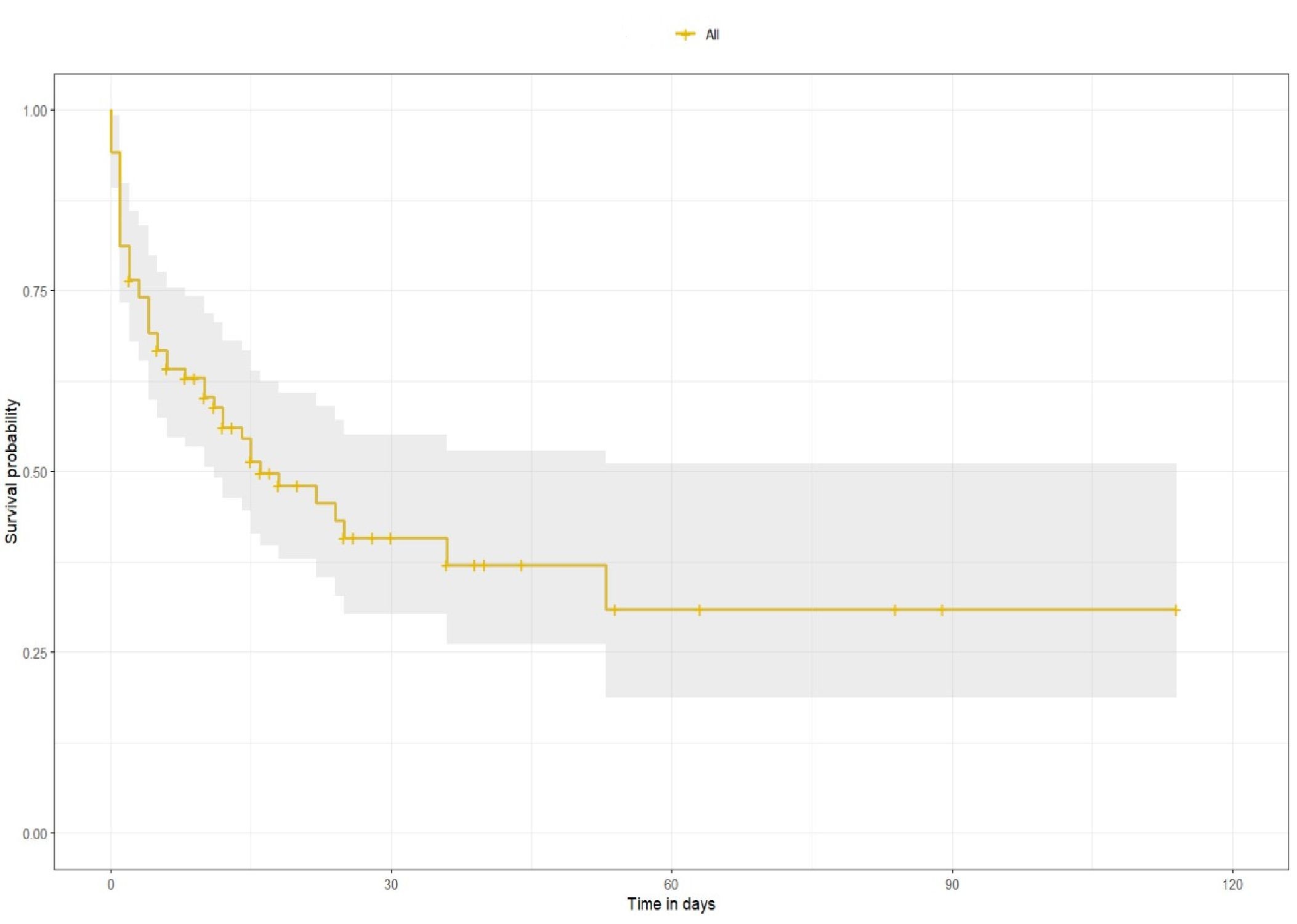
Figure 1. Overall survival after VA-ECMO implantation
VA-ECMO – Venoarterial extracorporeal membrane oxygenation
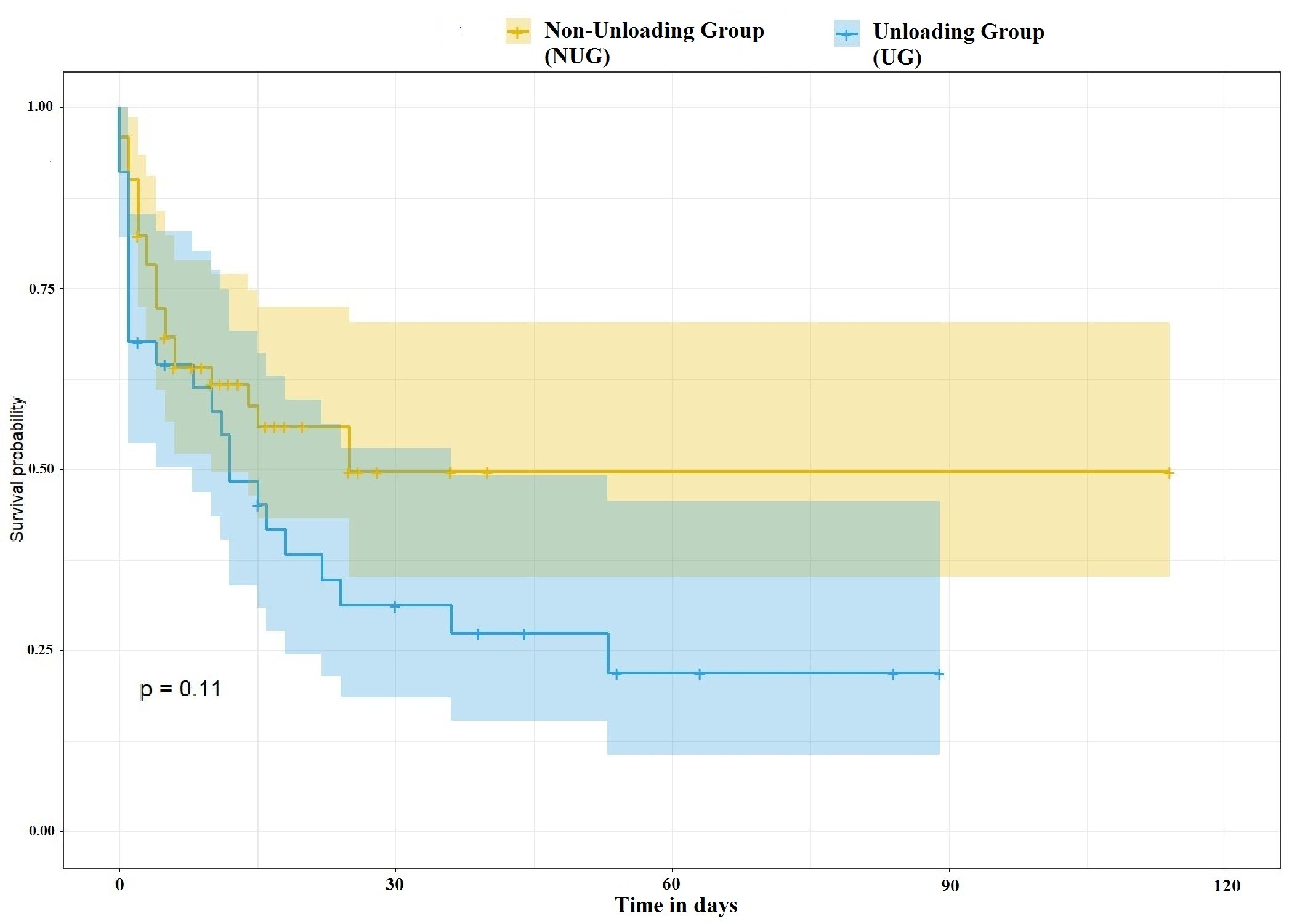
Figure 2. Survival after VA-ECMO implantation according to the use of unloading device
VA-ECMO – Venoarterial extracorporeal membrane oxygenation
Among patients who died, 37% died while on VA-ECMO, while 43% survived until hospital discharge or cardiac transplantation. The median duration of VA-ECMO support was 3 (0-52) days, and the median days of hospitalization was 11 (2-16) days. There were no major differences between the two groups regarding time on VA-ECMO support [4.0 (2.0, 6.0) days NUG vs. 5.0 (2.0, 8.0) days UG, p = 0.5] or in the duration of hospitalization [4.0 (3.0-8.0) days NUG vs. 5.0 (2.0-10.0) UG, p=0.8]. However, a greater proportion of patients survived until hospital discharge or transplantation in the NUG [28 patients (53.8%) in NUG vs. 9 patients (26.5%) in UG, p= 0.022] (Table 3).
|
Table 3. Survival rates |
|||||
|
Variables |
Study population (n = 86)1 |
Non-Unloading Group (NUG) (n = 52)1 |
Unloading Group (UG) (n = 34)1 |
p2 |
|
|
Days in VA-ECMO |
3.0 (0-52) |
4.0 (2.0-6.0) |
5.0 (2.0-8.0) |
0.5 |
|
|
11.0 (2-16.4) |
4.0 (3.0-8.0) |
5.0 (2.0-10.0) |
0.8 |
||
|
Alive to discharge or transplant, n(%) |
37 (43.0) |
28 (53.8) |
9 (26.5) |
0.022 |
|
|
Death in VA-ECMO, n(%) |
32 (37.2) |
17 (32.7) |
15 (44.1) |
0.399 |
|
|
SCAI |
Survival rate at 5 days (%) |
95% Confidence interval |
Survival rate at 30 days |
95% Confidence interval |
|
|
C |
0.100 |
|
0.729 |
(0.468-1.00) |
|
|
D |
0.726 |
(0.548-0.963) |
0.599 |
(0.406-0.884) |
|
|
E |
0.484 |
(0.336-0.696) |
0.213 |
(0.099-0.4458) |
|
|
1n (%); Median (IQR); 2Pearson's Chi-squared test; Wilcoxon rank sum test SCAI - Society for Cardiovascular Angiography and Interventions, VA-ECMO - venoarterial extracorporeal membrane oxygenation |
|||||
The 30-day survival rate significantly decreased with increasing severity of SCAI shock stage: SCAI C 72.9% (95% CI, 46.8-100%); SCAI D 59.9% (95% CI, 40.6-88.4%); SCAI E 21.3% (9.9-44.6%) (p=0.0035) (Table 3, Fig. 3).
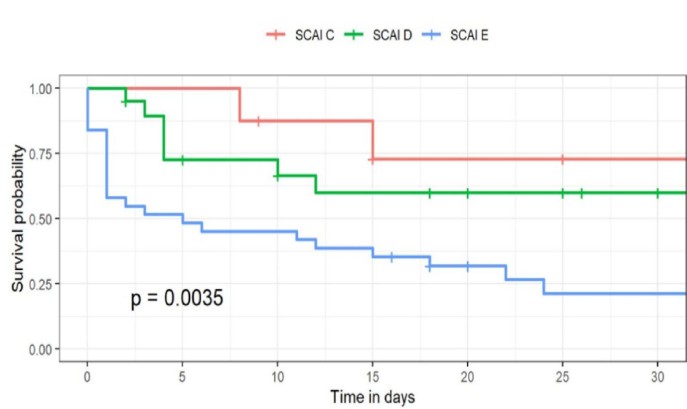
Figure 3. Survival rates according to SCAI stage of shock
SCAI - Society for Cardiovascular Angiography and Interventions
Multivariate analysis identified male gender (HR 2.99, 95% CI 1.29–6.98) and elevated lactate levels (HR 1.09, 95% CI 1.00–1.19) as significant predictors of 30-day mortality. Among the variables that did not reach statistical significance, cardiac arrest prior to VA-ECMO initiation (HR 1.22, 95% CI 0.41–3.65) and the use of E-CPR (HR 1.34, 95% CI 0.58–3.06) were associated with increased mortality (Figure 4).
After adjusting for the SCAI stage of shock, the use of LV unloading was not significantly associated with 30-day mortality (HR 1.21; 95% CI, 0.64–2.28). In contrast, SCAI stage E was independently associated with a significantly higher hazard of death (HR 2.24; 95% CI, 1.12–4.47).
Safety endpoints
Hematological conditions, such as anemia and thrombocytopenia requiring blood transfusions, were the most frequent VA-ECMO–related adverse events, occurring in 51.8% of patients. Bleeding complications were also significant, with 14.1% experiencing major vascular access-site bleeding, 17.6% gastrointestinal bleeding, 10.6% airway bleeding, and 2.4% intracerebral bleeding. Thrombotic and ischemic events were reported in 27.9% of patients (Table 4).
Airway bleeding events were significantly more prevalent in the UG (13 patients (3.8%) NUG vs. 15 patients (20.6%) UG, p=0.0369). Other bleeding events did not reach statistical significance between the two groups, although there was a trend toward a higher incidence of bleeding complications in the UG. Correspondingly, the UG received more red blood cell transfusions [1 (0-9) units in NUG vs. 3 (0-9) units in UG, p = 0.042]. There were no significant differences between groups regarding other device-related complications (Table 4).
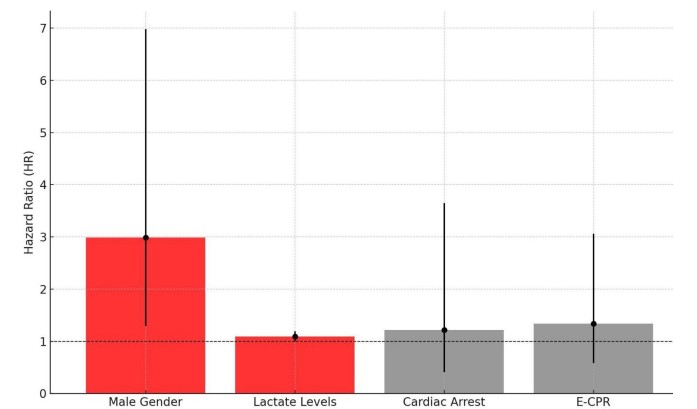
Figure 4. Mortality predictors
E-CPR – extracorporeal cardiopulmonary resuscitation
|
Table 4. VA-ECMO-related complications |
||||
|---|---|---|---|---|
|
Variables |
Study Population (n = 86)1 |
Non-Unloading Group (NUG) (n = 52)1 |
Unloading Group (UG) (n = 34)1 |
p2 |
|
Hematological complications, n(%)3 |
44 (51.8) |
13 (25) |
15 (44.1) |
0.12 |
|
Liver failure n(%)4 |
31 (36.0) |
19 (36.5) |
11 (32.4) |
0.817 |
|
Thromboembolic/Ischemic events, n(%)5 |
24 (27.9) |
11 (21.2) |
13 (38.2) |
0.752 |
|
Sepsis, n(%) |
23 (27.1) |
12 (23.1) |
11 (32.4) |
0.432 |
|
Gastrointestinal hemorrhage, n(%) |
15 (17.6) |
6 (11.5) |
9 (26.5) |
0.147 |
|
Access bleeding, n(%) |
12 (14.1) |
5 (9.6) |
7 (20.6) |
0.28 |
|
Airway bleeding, n(%) |
9 (10.6) |
2 (3.8) |
7 (20.6) |
0.0369 |
|
Intracerebral hemorrhage, n(%) |
2 (2.4) |
1 (1.9) |
1 (2.9) |
1.000 |
|
Red blood cells transfusion, Units, n(%) |
2 (0-9) |
1 (0-9) |
3 (0-9) |
0.042 |
|
Renal replacement therapy, n(%) |
27 (31.4) |
12 (23.1) |
15 (44.1) |
0.078 |
|
1n (%); Median (IQR); 2Pearson's Chi-squared test; Wilcoxon rank sum test; Fisher's exact test 3Requiring blood transfusions 4Defined as bilirubin ≥ 1.9 mg/dL or transaminases elevation > 70 UI/L; 5Venous and arterial thrombosis, acute limb ischemia, and compartment syndrome VA-ECMO – venoarterial extracorporeal membrane oxygenation |
||||
Discussion
Unloading analysis
In our cohort, the use of LV unloading was not associated with improved 30-day survival compared to patients who did not undergo active unloading, and fewer patients in the UG survived to hospital discharge or transplantation. After adjusting for shock severity, LV unloading remained unassociated with 30-day mortality, while male gender, higher baseline lactate, and SCAI stage E emerged as independent predictors of death.
No randomized studies have definitively established the benefits of unloading in VA-ECMO patients. From a physiological and hemodynamic perspective, unloading appears to be an advantageous strategy. However, the correlation with improved survival remains uncertain (11–13). During the SHOCK trial (2), the use of IABP for unloading was associated with improved short-term mortality [OR 0.82 (95% CI, 0.75–0.89), p<0.001] but also with an increased risk of major bleeding [OR 1.09 (95% CI, 1.0–1.18), p=0.03] when compared to VA-ECMO alone. Similarly, a meta-analysis conducted by Grajeda Silvestri et al. in 2020 (23), which included 448 patients, indicated a trend toward lower mortality in the group of VA-ECMO plus Impella® compared to those receiving VA-ECMO alone (52.6% vs. 63.6%, p < 0.01).
The unloading rates reported in the Extracorporeal Life Support in Cardiogenic Shock (ECLS-SHOCK) trial (5) were unexpectedly low (approximately 5.8%). In contrast, our cohort demonstrated an active unloading strategy in nearly 40% of VA-ECMO patients, with 76.5% of these interventions occurring in the 24 hours surrounding VA-ECMO implantation, as we anticipate adverse effects from VA-ECMO flow. High flow after VA-ECMO implantation often results in the failure of aortic valve opening, prompting the decision to early unloading (20).
Our results may be partially explained by the greater severity of illness in the UG, reflected in a significantly higher proportion of patients classified as SCAI stage E - 67.6% in UG vs. 44.2% in NUG (p = 0.04); worse analytical and hemodynamic parameters - mean bicarbonate 13.7 (5.25) mmol/L in UG vs. 18.0 (5.00) mmol/L in NUG (p = 0.01); mean lactate 12.5 (5.58) vs. 9.92 (5.88) mmol/L, (p = 0.315) and more frequent E-CPR - 41.2% UG vs. 28.8% NUG, (p = 0.342). This imbalance strongly suggests that the UG comprised a disproportionately higher-risk population as LV unloading tended to be employed in patients with more severe cardiogenic shock and higher perceived risk of LV distension - a consequence of clinical decision-making driven by perceived severity, not random allocation.
To address the imbalance in baseline severity between groups, we performed an adjusted survival analysis including SCAI stage as a covariate in a multivariable Cox model. This analysis confirmed that, after adjusting for baseline severity, the use of unloading devices was not independently associated with increased mortality (HR 1.21; 95% CI, 0.64–2.28). In fact, the higher crude mortality observed in the UG appears to be largely attributable to the overrepresentation of patients in SCAI stage E, which was confirmed as an independent predictor of death (HR 2.24; 95% CI, 1.12–4.47). These findings reinforce the hypothesis that the apparent lack of benefit with unloading may reflect selection bias toward sicker patients, rather than a true absence of efficacy. The fact that LV unloading did not worsen outcomes in a more critically ill group may support a potential non-inferiority signal, especially in selected high-risk patients.
The choice of unloading device at our center is based mostly on clinical experience, but also on individual patient characteristics, anatomical considerations, and device availability at the time of decision-making with a predominant use of IABP (67.6%) and, more recently, Impella CP®. Studies including over 90% of cases with IABP unloading have reported lower mortality rates (54%) compared to those receiving only VA-ECMO (65%), suggesting that IABP may suffice as a safe option for unloading (20, 21). While IABP unloading optimizes coronary perfusion, the Impella provides more robust unloading and can facilitate weaning from VA-ECMO and other advanced therapies (22, 23). In some cases, alternative forms of MCS were initiated prior to VA-ECMO support; once cannulation was performed, the original device was retained to serve as an unloading strategy.
Survival analysis
One key distinction between our cohort and those included in the ECLS-SHOCK trial (5) lies in the severity of cardiogenic shock. Approximately 55% of the patients presented in our cohort were classified as SCAI stage E, whereas the majority of patients in the ECLS-SHOCK trial were categorized as SCAI stage C (49.8%), reflecting a more favorable hemodynamic profile and laboratory parameters prior to VA-ECMO initiation (median lactate levels of 6.8 mmol/L in the ECLS-SHOCK trial vs. 11 mmol/L in our cohort) (5).
These parameters are well-established predictors of survival, as reflected in the SAVE score (16).
Our survival analysis suggests that patients in SCAI stage D may derive the greatest benefit from VA-ECMO implantation (Fig. 3). Although survival rates are predictably higher in SCAI stage C patients, initiating VA-ECMO in this group may expose them to complications that could potentially be avoided.
The survival curves for patients in SCAI stages D and E are closely aligned, with those in stage D exhibiting higher survival rates. Starting VA-ECMO support too early can lead to unnecessary complications associated with MCS, while waiting too long can render intervention futile. Also, prolonged cardiac arrest may result in anoxic encephalopathy or irreversible multi-organ failure, contributing to worse outcomes. Thus, timing in the management of cardiogenic shock is crucial (17).
Our multivariate analysis identified high lactate levels and male gender as significant predictors of mortality in patients undergoing VA-ECMO for cardiogenic shock. Men had a 2.99-fold higher risk of mortality compared to women, aligning with previous studies suggesting increased vulnerability of men in cardiogenic shock. Lactate levels emerged as a critical marker of disease severity with prognostic relevance, with each 1 mmol/L increase associated with a 9% higher risk of death. Although variables such as cardiac arrest prior to VA-ECMO and E-CPR did not reach statistical significance, their trends toward association with adverse outcomes highlight the need for further investigation.
VA-ECMO related complications
Bleeding complications were more frequent than thromboembolic events (34% vs. 28%, respectively) in our cohort, with vascular access site bleeding being the most common (14.1%). Notably, its incidence declined over the years as technical proficiency improved. Airway bleeding occurred in 10.6% of patients, the majority of whom required invasive mechanical ventilation (91.9%). This may be related to the need for systemic anticoagulation during VA-ECMO, which presents a continuous challenge in balancing thrombotic risk and bleeding. The critical condition of patients—often with severe cardiogenic shock and multi-organ dysfunction—may further contribute to vascular fragility and coagulopathy. Bleeding complications may also reflect specific institutional practices, including anticoagulation protocols and transfusion thresholds.
The introduction of additional MCS devices can increase the risk of bleeding and thrombotic events; however, a meta-analysis by Fiorelli et al. (25), found no significant difference in major bleeding (RR: 1.37; 95% CI: 0.88–2.13; p=0.16) or cerebrovascular accidents (RR: 0.91; 95% CI: 0.61–1.38; p=0.66) between VA-ECMO plus Impella® and VA-ECMO alone. In our cohort, a significantly higher incidence of airway bleeding was observed in the UG compared to the NUG (20.6% vs. 3.8%, p=0.0369). Although other types of bleeding did not differ significantly, they were also more frequent in unloaded patients and often required increased transfusion support.
Study limitations
Our study is limited by a small sample size and the retrospective nature of this analysis. The evaluation of unloading is further complicated by higher baseline severity of illness in the UG, which may have confounded outcome comparisons and introduced selection bias, as well as by the differing etiologies of cardiogenic shock between groups, given that distinct shock subtypes may respond differently to MCS. In cases of fulminant myocarditis, unloading—particularly with devices such as the Impella®—may confer a cardioprotective effect during phases of recovering LV systolic function (24). While this reflects real-world practice and enhances the external validity of our findings, the inclusion of diverse cardiogenic shock phenotypes and varied unloading techniques introduces a degree of clinical heterogeneity that may have influenced the outcomes. Additionally, 33.7% of our cohort underwent E-CPR prior to VA-ECMO implantation, a factor that has been consistently associated with poorer outcomes in previous studies (26).
Conclusion
In our cohort of patients supported with VA-ECMO, the 30-day survival rate was 41%, reinforcing its role as a potential rescue therapy in refractory cardiogenic shock. Although the use of LV unloading devices was not associated with improved 30-day survival, it did not appear to worsen outcomes in a population with greater baseline severity. Elevated lactate levels, male gender and SCAI E stage of shock emerged as independent predictors of mortality.
Ethics: This study was conducted in accordance with the Declaration of Helsinki. The authors declare that no experiments were conducted on humans or animals for this investigation.
Consent to participate / for publication: Patient data were retrospectively obtained and anonymised for this non-interventional study. The authors declare that no patient data appear in this article.
Peer-review: External and internal
Conflict of interest: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article
Authorship: M. L., F.N. and D.C. contributed to study conception, design and manuscript drafting; material preparation and data collection were performed by M.L., M.B., M.R.S., M.S., P. G. and M.P.;
F. N., M.B., G.P.-M. and D.C. contributed to data analysis and interpretation; A.D., P.B., D. C. and R.F.-C. assisted in clinical supervision. All authors read and approved the final version of the manuscript.
Acknowledgements : None
Funding: The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Statement on A.I.-assisted technologies use: Authors declare that they did not use AI-assisted technologies in preparation of this manuscript
Data and material availability: Data are available from the corresponding author on reasonable request.
In case authors share data, the fair use rules apply with acknowledgement of source/authors or collaboration
References
| 1.Thiele H, de Waha-Thiele S, Freund A, Zeymer U, Desch S, Fitzgerald S. Management of cardiogenic shock. EuroIntervention 2021; 17: 451-65. https://doi.org/10.4244/EIJ-D-20-01296 PMid:34413010 PMCid:PMC9724885 |
||||
| 2.Hochman JS,, Sleeper LA, Webb JG, Sanborn TA, White HD, Tailey JD, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock . New Engl J Med 1999; 341: 625-34. https://doi.org/10.1056/NEJM199908263410901 PMid:10460813 |
||||
| 3.Van Diepen S, Katz JN, Albert NM, Henry TD, Jacobs AK, Kapur NK, et al. Contemporary management of cardiogenic shock: a scientific statement from the american heart association. Vol. 136, Circulation. 2017. p. e232-68. 4.Møller JE, Engstrøm T, Jensen LO, Eiskjær H, Mangner N, Polzin A, et al. Microaxial flow pump or standard care in infarct-related cardiogenic shock. New Engl J Med 2024; 390: 1382-93. https://doi.org/10.1056/NEJMoa2312572 PMid:38587239 |
||||
| 5.Thiele H, Zeymer U, Akin I, Behnes M, Rassaf T, Mahabadi AA, et al. Extracorporeal life support in infarct-related cardiogenic shock. New Engl J Med 2023; 389: 1286-97. https://doi.org/10.1056/NEJMoa2307227 PMid:37634145 |
||||
| 6.Ostadal P, Rokyta R, Karasek J, Kruger A, Vondrakova D, Janotka M, et al. Extracorporeal membrane oxygenation in the therapy of cardiogenic shock: Results of the ECMO-CS randomized clinical trial. Circulation 2023; 147: 454-64. https://doi.org/10.1161/CIRCULATIONAHA.122.062949 PMid:36335478 |
||||
| 7.Abrams D, Combes A, Brodie D. Extracorporeal membrane oxygenation in cardiopulmonary disease in adults. J Am Coll Cardiol 2014; 63: 2769-78. https://doi.org/10.1016/j.jacc.2014.03.046 PMid:24814488 |
||||
| 8.Smith M, Vukomanovic A, Brodie D, Thiagarajan R, Rycus P, Buscher H. Duration of veno-arterial extracorporeal life support (VA ECMO) and outcome: An analysis of the Extracorporeal Life Support Organization (ELSO) registry. Crit Care 2017; 21: 45. https://doi.org/10.1186/s13054-017-1633-1 PMid:28264702 PMCid:PMC5339999 |
||||
| 9.Rastan AJ, Dege A, Mohr M, Doll N, Falk V, Walther T, et al. Early and late outcomes of 517 consecutive adult patients treated with extracorporeal membrane oxygenation for refractory postcardiotomy cardiogenic shock. J Thorac Cardiovasc Surg 2010; 139: 302-11. https://doi.org/10.1016/j.jtcvs.2009.10.043 PMid:20106393 |
||||
| 10.Combes A, Leprince P, Luyt CE, Bonnet N, Trouillet JL, Léger P, et al. Outcomes and long-term quality-of-life of patients supported by extracorporeal membrane oxygenation for refractory cardiogenic shock. Crit Care Med 2008; 36: 1404-11. https://doi.org/10.1097/CCM.0b013e31816f7cf7 PMid:18434909 |
||||
| 11.Truby LK, Takeda K, Mauro C, Yuzefpolskaya M, Garan AR, Kirtane AJ, et al. Incidence and implications of left ventricular distention during venoarterial extracorporeal membrane oxygenation support. ASAIO J 2017; 63: 257-65. https://doi.org/10.1097/MAT.0000000000000553 PMid:28422817 |
||||
| 12.Ezad SM, Ryan M, Donker DW, Pappalardo F, Barrett N, Camporota L, et al. Unloading the left ventricle in venoarterial ECMO: In whom, when, and how? Circulation 2023; 147: 1237-50. https://doi.org/10.1161/CIRCULATIONAHA.122.062371 PMid:37068133 PMCid:PMC10217772 |
||||
| 13.Soltes J, Rob D, Kavalkova P, Bruthans J, Belohlavek J. Growing Evidence for left ventricular Unloading in VA ECMO. J Clin Med 2023; 12: XXX-XX. https://doi.org/10.3390/jcm12186069 PMid:37763008 PMCid:PMC10531917 |
||||
| 14.Meani P, Lorusso R, Pappalardo F. ECPella: Concept, physiology and clinical applications. J Cardiothorac Vasc Anesth 2022; 36: 557-66. https://doi.org/10.1053/j.jvca.2021.01.056 PMid:33642170 |
||||
| 15.Passos Silva M, Caeiro D, Fernandes P, Guerreiro C, Vilela E, Ponte M, et al. Extracorporeal membrane oxygenation in circulatory and respiratory failure - A single‐center experience. Rev Port Cardiol 2017; 36: 833-42. https://doi.org/10.1016/j.repc.2017.01.003 PMid:29126895 |
||||
| 16.Schmidt M, Burrell A, Roberts L, Bailey M, Sheldrake J, Rycus PT, et al. Predicting survival after ECMO for refractory cardiogenic shock: The survival after veno-arterial-ECMO (SAVE)-score. Eur Heart J 2015; 36: 2246-56. https://doi.org/10.1093/eurheartj/ehv194 PMid:26033984 |
||||
| 17.Naidu SS, Baran DA, Jentzer JC, Hollenberg SM, van Diepen S, Basir MB, et al. SCAI SHOCK stage classification Expert Consensus Update: A Review and incorporation of validation studies. J Am Coll Cardiol 2022; 79: 933-46. https://doi.org/10.1016/j.jacc.2022.01.018 PMid:35115207 |
||||
| 18.Serebruany VL, Atar D. Assessment of bleeding events in clinical trials-proposal of a new classification. Vol. 99, Am J Cardiol 2007; 99: 288-90. https://doi.org/10.1016/j.amjcard.2006.07.091 PMid:17223436 |
||||
| 19.Daneshvar A, Mousa G. Regression shrinkage and selection via least quantile shrinkage and selection operator. PLoS One 2023;18: e0266267 https://doi.org/10.1371/journal.pone.0266267 PMid:36795659 PMCid:PMC9934385 |
||||
| 20. Yang F, Shen JZ, Lin XJ, Wang Z, Liu Y, Hao X, et al. Effects of intra-aortic balloon pump on cerebral blood flow during peripheral venoarterial extracorporeal membrane oxygenation support. J Transl Med 2014; 12: 106. https://doi.org/10.1186/1479-5876-12-106 PMid:24766774 PMCid:PMC4006449 |
||||
| 21.Aso S, Matsui H, Fushimi K, Yasunaga H. The Effect of intraaortic balloon pumping under venoarterial extracorporeal membrane oxygenation on mortality of cardiogenic patients: An analysis using a nationwide inpatient database. Crit Care Med 2016; 44: 1974-9. https://doi.org/10.1097/CCM.0000000000001828 PMid:27322361 |
||||
| 22.Schrage B, Sundermeyer J, Blankenberg S, Colson P, Eckner D, Eden M, et al. Timing of active left ventricular unloading in patients on venoarterial extracorporeal membrane oxygenation therapy. JACC Heart Fail 2023; 11: 321-30. https://doi.org/10.1016/j.jchf.2022.11.005 PMid:36724180 |
||||
| 23.Russo JJ, Aleksova N, Pitcher I, Couture E, Parlow S, Faraz M, et al. Left Ventricular Unloading During Extracorporeal Membrane Oxygenation in Patients With Cardiogenic Shock. J Am Coll Cardiol 2019; 73: 654-62. https://doi.org/10.1016/j.jacc.2018.10.085 PMid:30765031 |
||||
| 24.Spillmann F, Van Linthout S, Schmidt G, Klein O, Hamdani N, Mairinger T, et al. Mode-of-action of the PROPELLA concept in fulminant myocarditis. Eur Heart J 2019; 40: 2164-9. https://doi.org/10.1093/eurheartj/ehz124 PMid:30891599 PMCid:PMC6612367 |
||||
| 25.Fiorelli F, Panoulas V. Impella as unloading strategy during VA-ECMO: systematic review and meta-analysis. Rev Cardiovasc Med 2021; 22: 1503-11. https://doi.org/10.31083/j.rcm2204154 PMid:34957789 |
||||
| 26. Miraglia D, Ortiz C, Rached-D'Astorg E, Lelong B, Savary D, Boussen S, et al. Long-term neurologically intact survival after extracorporeal cardiopulmonary resuscitation: systematic review and meta-analysis. Resuscitation 2020; 4: 100045. https://doi.org/10.1016/j.resplu.2020.100045 PMid:34223320 PMCid:PMC8244502 |
||||
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

