Study of some biochemical and genetic markers in patients with congenital heart defects undergoing surgical correction: A case-control study
ORIGINAL RESEARCH ARTICLE
Study of some biochemical and genetic markers in patients with congenital heart defects undergoing surgical correction: A case-control study
Article Summary
- DOI: 10.24969/hvt.2025.599
- CARDIOVASCULAR DISEASES
- Published: 03/10/2025
- Received: 24/08/2025
- Revised: 21/09/2025
- Accepted: 22/09/2025
- Views: 1475
- Downloads: 682
- Keywords: Congenital heart defects, cardiolipin, Nox2, bone morphogenetic protein 4, lysocardiolipin acyltransferase, cytochrome b-245 beta chain, genetic polymorphisms, surgical outcomes, observational study
Address for Correspondence: Mohammed H. Hassan, Department of Medical Biochemistry, Faculty of Medicine, South Valley University, Qena 83523, Egypt
E-mail: Mohammedhosnyhassaan@yahoo.com, mohammedhosnyhassaan@med.svu.edu.eg
Phone: +201098473605
ORCID: Mohammed H. Hassan- 0000-0003-2698-9438; Ashraf Taye- 0000-0002-4018-6052; Ahmed Farouk-0000-0001-6381-793X; Mohamed Abdelbary-0000-0002-7734-9089; Marwa Okasha-0000-0003-2377-5303; Samer A. El-Sawy-0000-0002-9503-8241; Amira A. Abdelnaby-0009-0007-8687-1729; Ahmed Ghoneim-0009-0007-1973-7394;
Amal Hofni-0000-0001-5067-5047; Mohammed Farouk Abdel Hafez- 0009-0002-0058-9882
Mohammed H. Hassan1a*, Ashraf Taye1b, Ahmed Farouk2 , Mohamed Abdelbary1c, Marwa Okasha1a, Samer A. El-Sawy3, Amira A. Abdelnaby3, Ahmed Ghoneim2, Amal Hofni 1b, Mohammed Farouk Abdel Hafez2
1aMedical Biochemistry Department, Faculty of Medicine, 1b Pharmacology and Toxicology Department, Faculty of Pharmacy, and 1cCardiothoracic Surgery Department, Faculty of Medicine; South Valley University, Qena 83523, Egypt
2Cardiothoracic Surgery Department, Faculty of Medicine, Assiut University, Assiut, Egypt.
3Department of Restorative Dentistry and Basic Medical Sciences, Faculty of Dentistry, University of Petra, Amman 11196, Jordan
Abstract
Objective: Congenital heart defects (CHDs) represent structural malformations of the heart that appear at birth and impair its function. In nearly half of CHD cases, the precise pathogenic mechanisms remain unknown due to the complexity of their etiology. Nevertheless, multiple genetic determinants have been identified as contributors to CHD development. This study aimed to evaluate the possible associations of circulating biochemical markers (cardiolipin and NADPH oxidase 2, NOX2) and genetic profile of three single nucleotide polymorphisms (SNPs) in lysocardiolipin acyltransferase, bone morphogenetic protein 4 (BMP4), and NOX2 genes in CHD patients undergoing surgical correction and correlate them with clinical outcomes.
Methods: A case-control study was performed on 60 CHD patients (15 each with atrial septal defect, ventricular septal defect, patent ductus arteriosus and tetralogy of Fallot) and 40 healthy controls. Clinical data and echocardiography of all participants were collected. Serum levels of cardiolipin and Nox2 were detected utilizing high performance liquid chromatography “HPLC” and ELISA techniques respectively. BMP4 rs762642, LCLAT1 rs74357219, and cytochrome b-245 beta chain “CYBB” rs190924506 SNPs were analyzed via TaqManTM assays and real-time PCR.
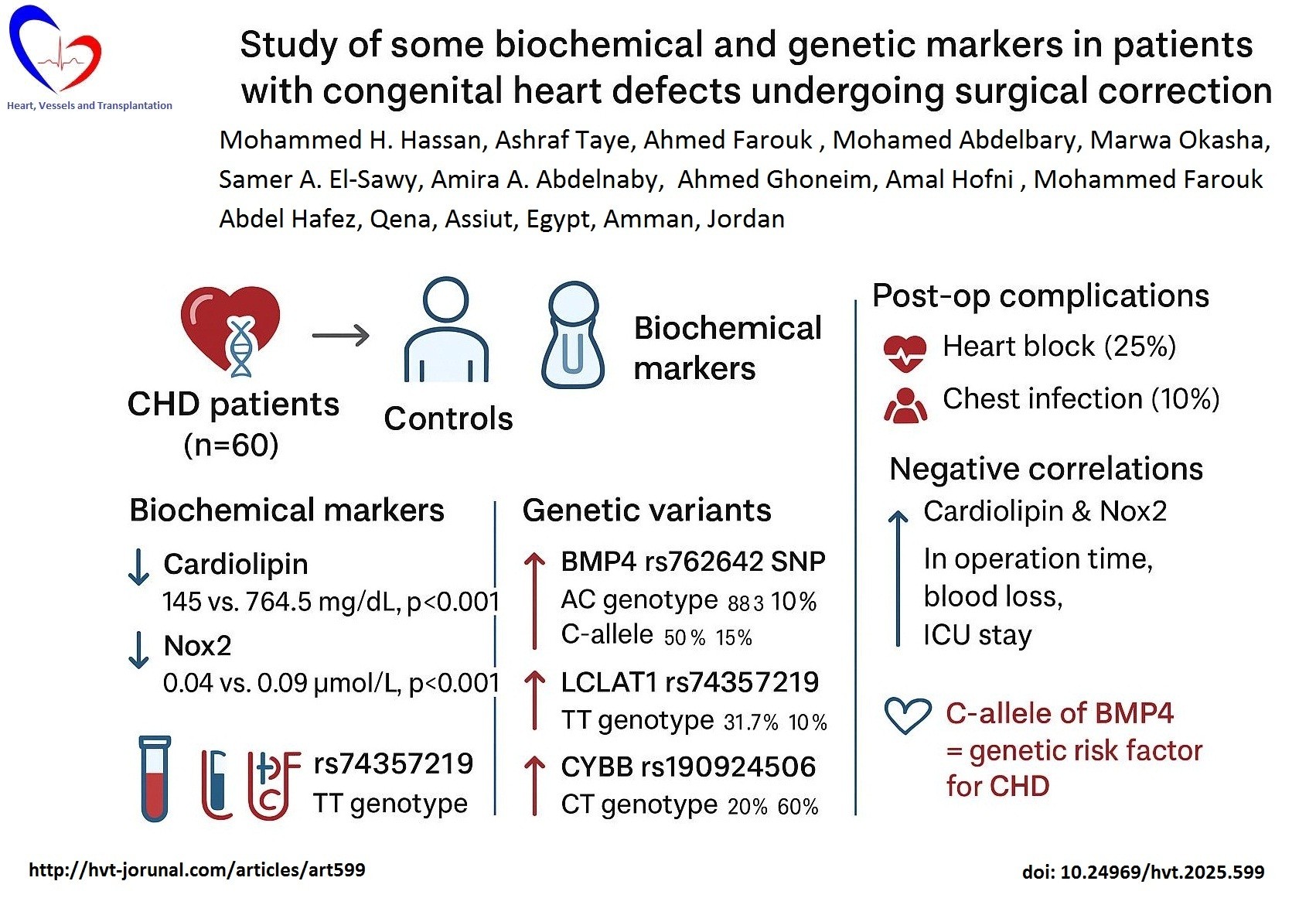
Results: CHD patients exhibited significantly lower cardiolipin (145 vs. 764.5 mg/dL, p<0.001) and Nox2 (0.04 vs. 0.09 µmol/L, p<0.001) compared to controls. Genetic analysis revealed dominance of AC genotype of BMP4 rs762642 SNP in CHD patients (83.3% vs. 10% in controls) and C-allele (50% vs. 15% in controls), p<0.001. The C-allele is regarded as a genetic risk factor for CHD with OR=0.333, 95%CI=0.179-0.620. There were higher frequency of TT genotype of LCLAT1 rs74357219 SNP (31.7% vs. 10%, p=0.014) and CT genotype of CYBB rs190924506 SNP (20% vs. 60%, p<0.001) in CHD patients relative to the controls with insignificant differences regarding their alleles frequencies. Postoperative complications included heart block (25%) and chest infection (10%). Cardiolipin and Nox2 correlated negatively with operation time, blood loss, and intensive care unit stay (p<0.05).
Conclusion: Reduced cardiolipin and NOX2 levels, along with BMP4, LCLAT1, and CYBB polymorphisms, are significantly associated with CHD and postoperative outcomes.
Key words: Congenital heart defects, cardiolipin, Nox2, bone morphogenetic protein 4, lysocardiolipin acyltransferase, cytochrome b-245 beta chain, genetic polymorphisms, surgical outcomes, observational study
Graphical abstract
Introduction
Congenital heart disease (CHD) represents the most common category of birth defects, encompassing a heterogeneous group of structural abnormalities such as atrial septal defect (ASD), ventricular septal defect (VSD), patent ductus arteriosus (PDA), and tetralogy of Fallot (TOF). While advances in surgical techniques and peri-operative management have improved survival, the underlying molecular mechanisms that drive these malformations and influence surgical outcomes remain incompletely understood. Identifying reliable biomarkers and genetic determinants could provide new insights into disease pathogenesis and improve pre-operative risk stratification (1, 2).
Mitochondrial dysfunction and oxidative stress have been increasingly implicated in cardiac development and performance. Cardiolipin (CL), a unique mitochondrial phospholipid, plays an essential role in oxidative phosphorylation, stabilizing electron transport chain complexes, and maintain energy metabolism. Its biological function largely depends on the composition of its acyl chains, which undergo marked alterations during the progression of age-associated disorders. Such pathological alterations in CL remodeling and acyl composition have been strongly linked to impaired myocardial bioenergetics and structural cardiac anomalies (3).
In the heart, nicotinamide adenine dinucleotide phosphate (NADPH) oxidases serve as a primary contributor to reactive oxygen species (ROS) generation. This multi-subunit enzyme complex facilitates the production of superoxide anions or hydrogen peroxide by channeling electrons from cytosolic NADPH to molecular oxygen. The typical NADPH oxidase (NOX) is composed of two membrane-bound subunits (gp91phox, also known as NOX2, and p22phox) together with three cytoplasmic components (p47phox, p67phox, and p40phox), in addition to the GTP-binding protein Rac. Despite this defined architecture, the precise contribution of NOX2 to cardiac development during embryogenesis is still unclear. It has been proposed that loss of NOX2 interferes with the endocardial-to-mesenchymal transition (EndMT), thereby leading to congenital malformations of the septum and valves (4). Together, these pathways connect mitochondrial lipid biology with redox homeostasis in CHD.
Bone morphogenetic proteins (BMPs), members of the transforming growth factor-β (TGF-β) superfamily, play a pivotal role in the early stages of cardiogenesis by directing mesodermal cell fate toward a cardiac lineage. BMP4 encodes a morphogen essential for cardiac septation and outflow tract formation, and its variants may predispose to structural CHD. During gastrulation, BMP signaling from the anterior endoderm and lateral plate mesoderm induces cardiac progenitor cells through activation of transcription factors like GATA4, Nkx2.5, and MEF2. BMP2 and BMP4, in particular, are essential for the specification of cardiac mesoderm, promoting myocardial differentiation while simultaneously inhibiting alternative mesodermal fates, such as hematopoietic or skeletal lineages. In later stages, BMPs contribute to endocardial cushion formation and valvulogenesis by regulating epithelial-to-mesenchymal transition (EMT) in the atrioventricular canal. Moreover, precise spatial and temporal regulation of BMP signaling is necessary, as excessive or deficient BMP activity may lead to congenital heart malformations, including septal defects and abnormal valve morphogenesis. Thus, BMP signaling acts as a critical morphogenetic cue integrating extracellular signals to orchestrate the complex process of heart development (5, 6).
The enzyme acyl-CoA: lysocardiolipin acyltransferase 1 (ALCAT1)—responsible for pathological remodeling of CL—was investigated for its impact on mitochondrial biogenesis. Evidence indicates that functional alteration in ALCAT1 expression induces mitochondrial fragmentation by promoting oxidative stress and could lead to defective mitochondrial lipid composition (7).
The CYBB gene, which encodes the catalytic subunit of NOX2, is responsible for producing NOX2, an enzyme that facilitates electron transfer from NADPH through FAD and heme to molecular oxygen inside the phagosome, affecting reactive oxygen species (ROS) generation and redox balance (8).
The hypothesis of the current study is to assess the possible contribution of variants in developmental (BMP4) and metabolic/redox genes (ALCAT1, and CYBB) to altered cardiolipin remodeling and NOX2 activity, which in turn influence CHD phenotype and perioperative outcomes (e.g. blood loss, operation time, hospital stay, and intensive care unit, ICU stay).
Methods
Study design and population
This cross-sectional case-control study was done at the Cardiothoracic Surgery Departments of Qena and Assiut University Hospitals in collaboration with the Medical Biochemistry Department, Faculty of Medicine, South Valley University, and included 60 patients (age range was 1 month -20 years; 21 males and 39 females) with congenital heart defects (15 with ASD, 15 with VSD, 15 with PDA, and 15 with TOF) in addition to 40 healthy participants (age range was 2 months – 10 years; 15 males and 25 females ) matched for age, sex, and body mass index who acted as the control group. The study period was from November 1st, 2021 to June 1st, 2024
Inclusion criteria: patients with congenital heart defect candidate for surgical repair of any age.
Exclusion criteria: patients who were participating in another study, those who denied or withdrew consent, patients with metabolic storage disorders or structural or numerical chromosomal abnormalities, those with a family history of dyslipidemia, individuals who refused to participate, patients with other comorbidities (renal, hepatic, or neurological diseases), and those with heart diseases other than congenital defects.
This study was approved by the Local Ethics Committee of the Faculty of Medicine, South Valley University (Approval Code: SVU-MED-MBC004-2-21-10-256). Written informed consent was obtained from all participants or their caregivers after a full explanation of the study's aims, in accordance with the Declaration of Helsinki.
Baseline variables
A full history and clinical examination were performed in all patients with special consideration to: age, sex, duration of the disease, onset, progress and symptoms, family history, and parental consanguinity; history of chronic diseases, including diabetes mellitus (DM) or hypertension; and medication history. Body mass index (BMI) computed by dividing the weight (kg) by square height m².
Echocardiography
Echocardiography was performed by an expert for the included cases to determine pulmonary artery pressure (PA), left ventricular end-systolic diameter (LVESD), left ventricular end-diastolic diameter (LVEDD), and fractional shortening (FS), using the GE Vivid S6 cardiac ultrasound (GE Medical Systems, Freiburg, Germany).
Surgery for CHD
All patients who were diagnosed with structural cardiac defects and required surgical intervention underwent operative correction. Surgical procedures were performed by experienced pediatric and adult cardiac surgeons under standardized operative and anesthetic protocols. The type of corrective surgery was determined based on preoperative echocardiographic findings, clinical status, and intraoperative assessment. Common operative procedures included atrial septal defect (ASD) closure, ventricular septal defect (VSD) closure, patent ductus arteriosus (PDA) ligation or division, tetralogy of Fallot (TOF) repair, and atrioventricular septal defect (AVSD) repair. Operative data including operation time, blood loss, type of operation, hospital stay days, and ICU stay days.
Blood samples collection
From all cases and control groups, 5 mL of venous blood was obtained after overnight fasting and divided as follows:
-2 mL whole blood in EDTA tubes: kept at -80 °C for later genetic analysis.
-3 mL in plain (serum gel separator) tubes: centrifuged at 3500 rpm for 15 minutes at 4 °C; serum was transferred into 1 mL cryotubes for later NOX2 and CL assays.
Specific biochemical assays
Serum GL levels analysis by high performance liquid chromatography (HPLC) was performed according to N.L. Parinandi (10) utilizing Agilent Technologies 1200 Series. Cardiolipin was extracted from serum by adding 300 μL of sample to 1 mL of chloroform, followed by 15 minutes of sonication. Samples were then centrifuged for 2 minutes at 500 rpm. Under specific chromatographic conditions, 50 μL of each sample was injected using a Zorbax Eclipse XDB C18 column (4.6 × 150 mm, 5-micron), analyzed with an isocratic elution of 100% methanol as the mobile phase at 1 mL/min flow rate at 40 °C, and detected by diode array detector (DAD) at 250 nm (9). The concentrations of CL were calculated using standard curves
Genetic assays
Genomic DNA Extraction and TaqMan™ single nucleotide polymorphism (SNP) genotyping assays were performed for the following polymorphisms:
• Bone morphogenetic protein 4 (BMP4-MIR5580) rs762642 A/C, a transversion substitution
• Lysocardiolipin acyltransferase 1 (LCLAT1) rs74357219 C/T, a transition substitution
• Cytochrome b-245 beta chain (CYBB) rs190924506 C/T, a transition substitution
a) DNA extraction from whole blood: Genomic DNA was extracted from each stored whole EDTA blood sample using the QIAamp DNA blood mini kit (Cat. No. 51104, QIAGEN, Germany), following the manufacturer’s protocol.
b) SNP genotyping assay: Genotyping of DNA samples was performed using quantitative real-time polymerase chain reaction (qRT-PCR) on the 7500 fast real-time PCR system (Applied Biosystems, USA).
Reaction preparation
For each SNP assay, the subsequent components were employed:
• 10 μL of TaqMan Genotyping Master Mix (Cat. No. 4371355, Thermo Fisher, USA; 2×)
• 5.0 μL of extracted DNA at a concentration of 10 ng/μL
• 1.0 μL of primer and probe assay (20×) for rs762642, rs74357219, and rs190924506 (Thermo Fisher, USA; with SNP IDs as shown in Table 1)
• 4.0 μL of nuclease-free water, resulting in a total volume of 20 μL per reaction with RNAse free water.
|
Table1. Gene names, polymorphisms, and assay ID of the studied SNPS |
|||
|
SNP ID |
rs762642 |
rs74357219 |
rs190924506 |
|
GENE |
BMP4-MIR5580 |
LCLAT1 |
CYBB |
|
GENE NAME |
bone morphogenetic protein 4 |
lysocardiolipin acyltransferase 1 |
cytochrome b-245 beta chain |
|
POLYMORPHISM |
A/C, Transversion substitution |
C/T, Transition substitution |
C/T, Transition substitution |
|
ASSAY ID |
C___ 3113065_10 |
C_103426526_10 |
C_186148261_10 |
|
|
TGCCCCGCACTTCCCAA AGGTGAGA[A/C]TCTC CCAGGGACTGCTGGAC AGAGA |
AGGGCAGTCTTGTTG GAAACCTTGC[C/T]CT TTAACTTGTGGGATCT GATACGA |
ATATAACAGTTTGTGAACAAAAAAT[C/T] TCAGAATGGGGAAAAATAAAGGAAT |
|
BMP4 - Bone morphogenetic protein 4, CYBB - cytochrome b-245 beta chain, ID – identity, LCLAT1 - lysocardiolipin acyltransferase 1, SNP – single nucleotide polymorphism |
|||
Statistical analysis
Sample size calculation
The study was computed utilizing GPower software version 3.1.3, with an alpha error probability of 0.05, power (1-beta error probability) of 0.80, and an allocation ratio of 1:1. The necessary sample size was 60 patients (15 patients in each group). The sample size was detected employing GPower software version 3.1.3. The subsequent simple formula was applied to determine the appropriate sample size in a prevalence study (9):
n = z²(1−p)/d²,
Where n is the sample size, Z is the standard normal variant (at 5% type error (p<0.05) it is 1.96), p is the expected prevalence, and d (absolute error or precision) = 0.05. The level of confidence usually aimed for is 95%.
Data were analyzed applying the Statistical Package for the Social Sciences (SPSS) version 27. A normality test, including Kolmogorov-Smirnov and Shapiro-Wilk tests, was performed and indicated that all data were not normally distributed. Continuous data were expressed as mean (standard deviation) for parametric data, or as median and interquartile range (Median [IQ]) for non-parametric data. Differences among two groups were analyzed employing the Mann–Whitney U test (U) for non-parametric data. For comparisons among three or more groups, the Kruskal–Wallis H test (H) was employed, as the variables were not normally distributed. Nominal data were expressed as percentages, and comparisons between two or more groups were conducted utilizing the Chi-square test (Chi).
For correlation coefficients, the Spearman test was applied due to non-parametric data distribution. Predictors of CHD in the studied patients were identified using a logistic regression model. The dependent variable was the presence of CHD (coded 1=present, 0=absent). Independent variables included serum cardiolipin, NOX2 , and the three studied SNPS (BMP4 rs762642, LCLAT1 rs74357219, and CYBB rs190924506). A two-tailed p-value < 0.05 was considered statistically significant.
Results
This cross-sectional case-control study was conducted on a total of 60 patients, divided into four categories (15 patients with ASD; 15 patients with VSD; 15 patients with PDA; 15 patients with TOF. These four patient groups were evaluated against a control group of 40 healthy age- and sex-matched volunteers.
Comparison of demographic and clinical data between CHD group and control group
Both groups exhibited a similar median age; CHD patients had an age of 3 years (age range was 1month -20 years) and 4 years in control group (age range was 2 months – 10 years). The gender distribution among the two groups demonstrated no significant difference with female predominance: 35% males (21 patients) and 65% females (39 patients) in the CHD group and 37.5% (15 patients) for male and 62.5% for female (25 patients) in control group. The median weight was 14.75 kg for CHD nonsignificantly lower than median of 15.5 kg for control. The BMI comparison showed a median of 17.32 kg/m2 for CHD patients while 16.48 kg/m2 in control. The CHD cases were matched with controls for age, gender, weight, and BMI, as revealed by the insignificant differences among the two groups (p>0.05 for all), while it shows significant difference regarding the height with median of 86 cm for CHD patients much lower than median of control 99 cm (p=0.039).
Comparison of clinical, echocardiographic and intraoperative data (Table 2)
|
Table 2. Clinical, echocardiographic and intraoperative data of the study groups |
|||
|
Variables |
Cases (n=60) |
Controls (n=40) |
p |
|
Type of congenital heart disease, n(%) |
|||
|
PDA |
15 (25) |
- |
- |
|
VSD |
15 (25) |
- |
- |
|
ASD |
15 (25) |
- |
- |
|
TOF |
15 (25) |
- |
- |
|
Family history, n(%) |
|||
|
Negative |
36 (60) |
35 (87.5) |
0.001* |
|
Positive |
24 (40) |
5 (12.5) |
- |
|
Parental consanguinity, n(%) |
|||
|
Negative |
25 (41.7) |
32 (80) |
0.002* |
|
Positive |
35 (58.3) |
8 (20) |
|
|
Type of operation, n(%) |
|||
|
Open sternotomy |
45 (75) |
- |
- |
|
Thoracotomy |
15 (25) |
- |
- |
|
Operation time, min |
200 (147–282.5) |
- |
- |
|
Blood loss, cc |
450 (300–600) |
- |
- |
|
ICU stay, days |
1 (1–2) |
- |
- |
|
Hospital stay, days |
6 (5–7) |
- |
- |
|
LVEDD, mm |
29 (26.85–33) |
27 (25–28) |
0.002 |
|
LVESD, mm |
26.15 (22.88–28) |
22 (20–24) |
0.001 |
|
Pulmonary artery pressure, mmHg |
16 (15–17) |
12 (11–14) |
<0.001 |
|
FS, % |
40 (37.25–43) |
45 (43–48) |
<0.001 |
|
Data are presented as number (percentage)and median (interquartile range) Chi-square and Mann- Whitney U tests ASD - atrial septal defect, FS – fraction shortening, ICU – intensive care unit, LVEDD-- left ventricular end-diastolic dimension, LVESD – left ventricular end-systolic dimension, PDA - patent ductus arteriosus, TOF - tetralogy of Fallot, VSD - ventricular septal defect |
|||
As can be seen from Table (2), patient group included 60 cases (15 VSD, 15 PDA, 15 ASD, and 15 TOF), while the control group had no CHD. A positive family history of similar cardiac conditions was present in 40% of cases versus 8.3% of controls (p=0.001). Parental consanguinity was reported in 58.3% of cases compared to 20% of controls (p=0.002). Among cases, 75% underwent open sternotomy and 25% thoracotomy.
The median operation time was 200 min, with a median blood loss of 450 cc. Median ICU stay was 1 day, and hospital stay was significantly longer in cases (median 6 days) compared to controls (median 3 days; p<0.001). Preoperative echocardiography in cases showed a median LVEDD of 29 mm, LVESD of 26.15 mm, and pulmonary artery pressure of 16 mm, all significantly greater than in controls (p<0.01), while median FS was lower in cases (40%) than controls (45%) (p<0.001).
Concerning the outcome of the examined group, it was 10% (6 cases) of patients with CHD had chest infection, and 25% (15 cases) of them had heart block, 1.6% (1 case) died, while 63.4%(38 cases) of them did not have postoperative complications.
Comparison of biochemical markers
As can be seen from Table 3, cardiolipin median levels were significantly reduced in patients (145 mg/dl) than in controls (764 mg/dl) (p=0.001). NOX2 median levels were also lower in patients (0.04 µmol/l) relative to controls (0.09 µmol/l) (p<0.001).
|
Table 3. Serum cardiolipin and NOX2 concentrations among the study groups |
|||
|
Variables |
Cases (n=60) |
Control (n=40) |
p |
|
Cardiolipin concentration, mg/dl |
|||
|
Min.-Max. |
25 - 2058.5 |
690 – 1173 |
|
|
Median(Q1-Q3) |
145(93.75-299.75) |
764.5(740.5-952.5) |
<0.001 |
|
NOX2, µmol/l |
|||
|
Min.-Max. |
0.01 - 0.78 |
0.06 - 0.24 |
|
|
Median(Q1-Q3) |
0.04(0.03-0.07) |
0.09(0.07-0.17) |
<0.001 |
|
Data are presented as minimum –maximum and median (interquartile range) Mann- Whitney U test NOX2 - nicotinamide adenine dinucleotide phosphate oxidase 2 |
|||
Table 4 presents cardiolipin and NOX2 levels among types of CHD groups and controls. Cardiolipin level was the lowest in TOF (53 mg/dl), compared to 170 mg/dl (ASD), 128 mg/dl (VSD), 1153 mg/dl (PDA), and 764.5 mg/dl (control) ( p<0.001). NOX2 showed similar results with the lowest level in TOF (0.03 mmol/l), followed by 0.04 mmol/l in VSD and ASD, and 0.09 mmol/l in both PDA and control (p<0.001).
|
Table 4. Comparison of cardiolipin and NOX2 concentrations between CHD types groups and controls |
||||||
|
Variables |
Type of congenital heart disease |
Control group |
p |
|||
|
PDA |
VSD |
ASD |
TOF |
|||
|
n=15 |
n=15 |
n=15 |
n=15 |
n=40 |
||
|
Cardiolipin, mg/dl |
||||||
|
Min.-Max. |
311 - 2058.5 |
114 – 266 |
114 - 240 |
25 – 87 |
690 – 1173 |
|
|
Median(Q1 -Q3) |
1153(540- 1300) |
128(119- 223) |
170(119- 230) |
53(47-56) |
764.5(740- 952.5) |
<0.001*
|
|
NOX2, µmol/l |
||||||
|
Min.-Max. |
0.04 – 0.78 |
0.02 – 0.07 |
0.03 – 0.05 |
0.01 – 0.08 |
0.06 - 0.24 |
|
|
Median(Q1 -Q3) |
0.09(0.07- 0.23) |
0.04(0.03- 0.05) |
0.04(0.03- 0.05) |
0.03(0.02- 0.04) |
0.09(0.07- 0.17) |
<0.001*
|
|
Data are presented as minimum –maximum and median (interquartile range) Mann- Whitney U test ASD - atrial septal defect, CHD – congenital heart disease, NOX2 - cytochrome b-245 beta chain complex, PDA - patent ductus arteriosus, TOF - tetralogy of Fallot, VSD - ventricular septal defect |
||||||
Comparison of genetic markers
Table 5 explores genetic differences between CHD patients and controls by analyzing BMP4-MIR5580 A/C (rs762642) genotype and allele frequencies. A highly significant difference was observed in the overall genotype distribution between the two groups (p<0.001). The heterozygous AC genotype was more frequent in patients (83.3%) compared with controls (10%), conversely, the homozygous CC genotype was present in 8.3% of patients versus 20% of controls (p<0.001). When genotype combinations were considered, carriers of (AA+AC) showed a non-significant protective effect against disease (OR=2.750, 95% CI: 0.829- 9.124, p=0.089), while AC+CC carriers were significantly overrepresented in patients (91.7% vs. 30%). At the allele level, the A allele frequency was much lower in patients (50%) compared with controls (75%), whereas the C allele was substantially more frequent in patients (50%) than controls (15%), (p<0.001), further supporting a strong association between the C allele and disease susceptibility with (OR=0.333, 95% CI: 0.179- 0.620).
|
Table 5. Genotypes and alleles frequencies of BMP4 gene (rs762642) SNP among the studied groups |
|||||||||
|
Groups |
BMP4 rs762642 genotypes (n, %) |
BMP4 rs762642 alleles (n, %) |
|||||||
|
AA |
AC |
CC |
AA+AC |
CC |
AC+CC |
AA |
A |
C |
|
|
Patients (n=60) |
5(8.3) |
50(83.3) |
5(8.3) |
55 (91.7) |
5 (8.3) |
55 (91.7) |
5(8.3) |
60(50) |
60 (50) |
|
Controls (n=40) |
28(70) |
4(10) |
8(20) |
32(80) |
8(20) |
12(30) |
28(70) |
60(75) |
20(15) |
|
p (Chi-square)* |
<0.001(54.071) |
0.089 (2.888) |
<0.001(41.278) |
<0.001(12.500) |
|||||
|
OR (95%CI)* |
- |
2.750(0.829-9.124) |
25.667(8.224-80.108) |
0.333(0.179-0.620) |
|||||
|
Data are presented as number (percentage) Chi-square test and logistic regression analysis BMP4 - bone morphogenetic protein 4, CI – confidence interval, OR – odds ratio, SNP – single nucleotide polymorphism |
|||||||||
Table 6 explores genetic disparities between CHD patients and controls by analyzing LCLAT1 C/T (rs74357219) genotype and allele frequencies. The heterozygous CT being more frequent in controls (50%)
compared to patients (26.7%), the CC genotype was present at comparable frequencies in patients (41.7%) and controls (40%), and the TT genotype, however, was more frequent in patients (31.7%) than controls (10%), (p=0.014).
|
Table 6. Genotypes and alleles frequencies of LCLAT1 rs74357219 SNP within the studied groups |
|||||||||
|
Groups |
LCLAT1 rs74357219 Genotypes (n, %) |
LCLAT1 rs74357219 alleles (n, %) |
|||||||
|
CC |
CT |
TT |
CC+CT |
TT |
CT+TT |
CC |
C |
T |
|
|
Patients (n=60) |
25(41.7) |
16(26.7) |
19(31.7) |
41(68.3) |
19(31.7) |
35(58.3) |
25(41.7) |
66(55) |
54(45) |
|
Controls (n=40) |
16(40) |
20(50) |
4(10) |
36(90) |
4(10) |
24(60) |
16(40) |
52(65) |
28(35) |
|
p (Chi square) |
0.014(8.544) |
0.012(6.362) |
0.868(0.028) |
0.159(1.984) |
|||||
|
OR (95% CI) |
- |
0.240(0.075-0.771) |
0.933(0.413-2.108) |
0.658(0.367-1.179) |
|||||
|
Data are presented as number (percentage) Chi-square test and logistic regression analysis CI – confidence interval, LCLAT1 - lysocardiolipin acyltransferase 1, OR – odds ratio, SNP – single nucleotide polymorphism |
|||||||||
Using dominant model, genotype combinations (CC+CT vs. TT) showed significantly higher TT frequency among cases vs. controls (31.7 %, 10%, respectively) while, CC+CT genotypes were higher among the controls compared to cases (90% vs. 68.3%, with p=0.012, indicating that TT genotype could be regarded as genetic risk factor for development of CHD with OR=0.240 (95% CI=0.075-0.771). At the allele level, the C allele was more prevalent among controls (65%) compared to patients (55%), while the T allele was relatively more frequent in patients (45% vs. 35% in controls). Although this suggests that the T allele may contribute to increased disease susceptibility, the association did not achieve statistical significance in this dataset.
Table 7 explores genetic disparities between CHD patients and controls by analyzing CYBB (rs74357219) genotype and allele frequencies. There was a highly significant difference in the overall genotypes distribution between the two groups (p<0.001). The heterozygous CT genotype was markedly more frequent in controls (57.5%) compared to patients (20%), the homozygous TT genotype appeared predominantly in patients (30%) versus only 2.5% of controls, while CC genotype was incomparable between cases (50%) and controls (40%). Using dominant model, combined genotype (CC+CT) was significantly frequent among controls (97.5%) compared to cases (70%), p<0.001 (OR =0.060and 95% CI 0.008-0.470), confirming that TT genotypes could be regarded as a genetic risk factor for CHD development. At the allele level, the C allele was more common in controls (68.8%) than in patients (60%), whereas the T allele was more prevalent in patients (40% vs. 31.3%). Although this suggests a possible association of the T allele with increased disease susceptibility, the difference did not reach statistical significance in this dataset.
|
Table 7. Genotypes and alleles frequencies of CYBB rs190924506 SNP within the studied groups |
|||||||||
|
Groups |
CYBB rs190924506 Genotypes (n, %) |
CYBB rs190924506 alleles (n, %) |
|||||||
|
CC |
CT |
TT |
CC+CT |
TT |
CT+TT |
CC |
C |
T |
|
|
Patients (n=60) |
30(50) |
12(20) |
18(30) |
42(70) |
18(30) |
30(50) |
30(50) |
72(60) |
48 (40) |
|
Controls (n=40) |
16(40) |
23(57.5) |
1(2.5) |
39(97.5) |
1(2.5) |
24(60) |
16(40) |
55(68.75) |
25 (31.25) |
|
p (Chi-square) |
<0.001(23.188) |
<0.001(14.634) |
0.966(0.326) |
0.149(2.083) |
|||||
|
OR (95%C I) |
- |
0.060(0.008-0.470) |
0.667(0.297-1.499) |
0.643(0.352-1.173) |
|||||
|
Data are presented as number (percentage) Chi-square test and logistic regression analysis CI – confidence interval, CYBB - cytochrome b-245 beta chain , OR – odds ratio, SNP – single nucleotide polymorphism |
|||||||||
Table 8 delves into the interplay between BMP4 rs762642 genotypes and serum levels of cardiolipin and NOX2, key biomarkers in CHD. Cardiolipin levels varied by genotype: (53 mg/dL in AC, 119 mg/dL in CC, and 177.5 mg/mL in AA (p=0.004). NOX2 levels showed no significant differences between genotypes (p = 0.743). For LCLAT1 rs74357219, CL levels showed a marked genotype-dependent variation (p<0.001), being highest in CT carriers (1146 mg/dL; 255.68–1196.88) compared to CC (119 mg/dL; 59–146.5) and TT (119 mg/dL; 56–230.1). NOX2 levels also differed significantly (p<0.001), with CT genotypes showing the highest median (0.08 µmol/L; 0.07–0.098) versus CC and TT (both medians 0.04 µmol/L; 0.03–0.05). For CYBB rs190924506, CL n levels were significantly elevated in CT carriers (1168.5 mg/dL; 1143–1375) compared to CC (117 mg/dL; 54.75–173.75) and TT (128 mg/dL; 116.25–238.08) (P < 0.001). NOX2 levels also varied significantly by genotype (p< 0.001), with TT genotypes showing the highest median (0.07 µmol/L; 0.05–0.083) compared to CC (0.04 µmol/L; 0.03–0.05) and CT (0.03 µmol/L; 0.02–0.19).
|
Table 8. Comparison of serum cardiolipin and NOX2 concentrations between different genotypes of BMP4 rs762642, LCLAT1 rs74357219, and CYBB rs190924506 SNPs among patients with CHD (n=60) |
|||||||||
|
Variables
|
BMP4 rs762642 SNP |
*p |
|||||||
|
AA |
AC |
CC |
|||||||
|
n=5 |
n=50 |
n=5 |
|||||||
|
Cardiolipin, mg/dl |
177.5(116.75-375.3) |
53(37.4-70.5) |
119(59.5-196) |
0.004 |
|||||
|
NOX2, µmol/l |
0.04(0.02-0.07) |
0.04(0.03-0.07) |
0.05(0.03-0.06) |
0.743 |
|||||
|
|
LCLAT1 rs74357219 SNP |
*p |
|||||||
|
CC |
CT |
TT |
|||||||
|
n=25 |
n=16 |
n=19 |
|||||||
|
Cardiolipin concentration, mg/dl |
119(59-146.5) |
1146(255.68-1196.88) |
119(56-230.1) |
<0.001 |
|||||
|
NOX2, µmol/l |
0.04(0.03-0.05) |
0.08(0.07-0.098) |
0.04(0.03-0.05) |
<0.001 |
|||||
|
|
CYBB rs190924506 SNP |
*p |
|||||||
|
CC |
CT |
TT |
|||||||
|
n=30 |
n=12 |
n=18 |
|||||||
|
Cardiolipin concentration, mg/dl |
117(54.75-173.75) |
1168.5(1143-1375) |
128(116.25-238.08) |
<0.001 |
|||||
|
NOX2, µmol/l |
0.04(0.03-0.05) |
0.03(0.02-0.19) |
0.07(0.05-0.083) |
<0.001 |
|||||
|
Data are presented as Median (Q1--Q3) *Kruskal-Wallis H test BMP4 - Bone morphogenetic protein 4, CHD – congenital heart disease, CYBB - cytochrome b-245 beta chain, LCLAT1 - lysocardiolipin acyltransferase 1, NOX2 - nicotinamide adenine dinucleotide phosphate oxidase 2, SNP – single nucleotide polymorphism |
|||||||||
Correlation between biochemical markers and clinical variables in CHD
Regarding the correlations between cardiolipin, NOX2 levels, and various parameters among the included patients, CL demonstrated a strong positive correlation with NOX2 (r = 0.665, p < 0.001) and type of operation (r = 0.750, p < 0.001), and a moderate positive correlation with BMI (r = 0.267, p = 0.039). Significant negative associations were found with operation time (r = –0.854, p < 0.001), blood loss (r = –0.769, p < 0.001), age (r = –0.395, p = 0.002), weight (r = –0.361, p = 0.005), height (r = –0.407, p = 0.001), ICU stay (r = –0.573, p < 0.001), hospital stay (r = –0.414, p = 0.001), and FS% (r = –0.527, p < 0.001).
NOX2 levels displayed strong positive correlation with type of operation (r = 0.686, p < 0.001), and strong negative associations with operation time (r = –0.636, p < 0.001) and blood loss (r = –0.668, p < 0.001). Moderate negative correlations were observed with age (r = –0.323, p = 0.012), weight (r = –0.343, p = 0.007), height (r = –0.342, p = 0.007), ICU stay (r = –0.399, p = 0.002), and FS% (r = –0.479, p < 0.001). No significant correlations were noted between either cardiolipin or NOX2 with sex, family history, parental consanguinity, echocardiographic parameters, or outcome (all p > 0.05).
Association of biochemical and markers with CHD
Regarding univariate and multivariate regression analysis of predictors of CHD, high CL levels were positive predictors (protective), while AC carrier of BMP4 was a negative predictor (p<0.05). Univariate analysis also showed that high NOX2 levels, AA carrier of BMP4, CT carrier of LCAT, and CT carrier of CYBB were protective, whereas AC carrier of BMP4 and TT carrier of LCAT were negative predictors (p < 0.05 for all), (Table.9).
|
Table 9. Univariate and multivariate regression analysis for predictors of congenital heart diseases |
||||||||||
|
Variables |
Univariate analysis |
Multivariate analysis |
||||||||
|
B |
p |
Exp (B) |
95% CI for EXP(B) |
B |
p |
Exp(B) |
95% CI for EXP(B) |
|||
|
Lower |
Upper |
Lower |
Upper |
|||||||
|
Cardiolipin, mg/dl |
0.003 |
<0.001
|
1.003 |
1.002 |
1.004 |
0.006 |
<0.001
|
1.006 |
1.002 |
1.009 |
|
NOX2, µmol/l |
6.955 |
0.035 |
1048. 701 |
1.608 |
68406 4.357 |
7.032 |
0.100 |
1132.1 12 |
0.262 |
48866 29 |
|
BMP4 rs762642 SNP |
||||||||||
|
AA |
3.245 |
<0.001 |
25.66 7 |
8.224 |
80.108 |
1.018 |
0.435 |
2.769 |
0.215 |
35.683 |
|
AC |
-3.807 |
<0.001
|
0.022 |
0.006 |
0.076 |
-4.398 |
0.003 |
0.012 |
0.001 |
0.228 |
|
LCLAT1 rs74357219 SNP |
||||||||||
|
CT |
1.012 |
0.019 |
2.750 |
1.183 |
6.393 |
0.773 |
0.598 |
2.167 |
0.122 |
38.416 |
|
TT |
-1.428 |
0.017 |
0.240 |
0.075 |
0.771 |
-2.539 |
0.077 |
0.079 |
0.005 |
1.320 |
|
CYBB rs190924506 SNP |
||||||||||
|
CT |
1.792 |
<0.001
|
6.000 |
2.453 |
14.678 |
-1.306 |
0.362 |
0.271 |
0.016 |
4.500 |
|
Logistic regression analysis BMP4 - Bone morphogenetic protein 4, CHD – congenital heart disease, CI – confidence interval, CYBB - cytochrome b-245 beta chain, LCLAT1 - lysocardiolipin acyltransferase 1, NOX2 - nicotinamide adenine dinucleotide phosphate oxidase 2, SNP – single nucleotide polymorphism |
||||||||||
Discussion
CHD remains the most prevalent birth defect across the globe, affecting approximately 1% of live births. Despite significant advances in diagnostic and therapeutic approaches, CHD remains a primary cause of infant mortality and childhood morbidity. Recent research has increasingly focused on elucidating the molecular mechanisms underlying CHD pathogenesis, particularly the roles of mitochondrial dysfunction and oxidative stress pathways (11).
The emerging understanding of mitochondrial involvement in CHD has highlighted CL deficiency as a potential key factor in disease pathogenesis. CL, a phospholipid uniquely localized to the inner mitochondrial membrane is essential for preserving both mitochondrial structure and function. Studies have demonstrated significant alterations in CL profiles in children with severe CHD subtypes, particularly tetralogy of Fallot (TOF) (12).
Our study investigated the role of biochemical markers (CL, NOX2) and genetic polymorphisms (BMP4, LCLAT1, CYBB) in CHD, focusing on their diagnostic potential, association with disease severity, and surgical outcomes. The findings highlight significant alterations in mitochondrial and oxidative stress pathways, with strong genetic contributions, offering new insights into CHD pathophysiology and potential clinical applications.
Our results showed that well-matched controls (age, sex, BMI) ensure comparability. CHD patients had significantly shorter height (p=0.039), possibly due to growth retardation from chronic cardiac stress.
In agreement with our results regarding shorter height in CHD patients (p=0.039), a meta-analysis by Massaro et al. (13) found that CHD patients had significantly lower height-for-age Z-scores (−0.5 to−1.5) compared to healthy controls. Studies showed that children with CHD, especially cyanotic defects (e.g., TOF), often exhibit growth retardation due to chronic hypoxia, increased metabolic demands, and poor nutrition (13).
Against our results, several investigations have indicated no significant height differences in mild CHD (e.g., small ASD/VSD). A study demonstrated that children with mild CHD (small ASDs/VSDs) showed normal growth patterns, height and weight z-scores were comparable to healthy controls, supporting the contrasting evidence that not all CHD subtypes affect growth (14).
Our study revealed a significant connotation between CHD and positive family history, with 40% of cases reporting affected relatives compared to only 8.3% of controls (p = 0.001). Parental consanguinity was observed in 58.3% of cases, significantly greater than in controls (20%, p=0.002), reinforcing the established link between consanguinity and CHD risk. These findings align with the findings of Blue et al. (15) who noted that familial clustering is especially prominent in septal defects and conotruncal malformations, both of which were common in our cohort.
In terms of postoperative outcomes, chest infection (10%) and complete heart block (25%) were the most frequent complications in our cohort. These rates are comparable to those reported in pediatric cardiac surgery literature where conduction disturbances and pulmonary complications remain notable contributors to morbidity despite advances in surgical techniques and perioperative care (16).
In our study, CHD patients exhibited significantly lower serum levels of both CL and NOX2, indicating a marked biochemical alteration associated with CHD development and pathology. In accordance with our results, a previous study by Garcia et al. (17) has observed depletion of CL content in myocardial tissue of children with single-ventricle heart failure a phenotype related to congenital heart malformations. This reduction was accompanied by compensatory upregulation of CL biosynthetic and remodeling enzymes. These findings align with our observation of lower circulating CL levels in CHD patients, suggesting mitochondrial phospholipid dysregulation (17).
While elevated NOX2 activity is often linked to oxidative stress in pathological states like heart failure, genetic studies in mice reveal that NOX2 deficiency disrupts normal embryonic heart development (e.g., septal and valvular defects) via impaired EndMT (18). However, our findings of reduced NOX2 a ROS-producing enzyme suggest a more complex interplay rather than peripheral ROS overdrive, CHD may involve impaired ROS signaling elements during cardiogenesis or mitochondrial adaptation. In disagreement with our results, a meta-analysis by Vanreusel et al. (19) emphasized that patients with CHD exhibit elevated oxidative stress, often related to mitochondrial dysfunction and ROS overproduction (19).
Our study presented a detailed analysis of biochemical markers (CL and NOX2) and genetic ploymorphisms (BMP4 (rs762642), ALCAT1 (rs74357219) and CYBB (rs190924506)) in patients with CHD, comparing them to healthy controls.
Our study noted that CL was significantly lower in CHD compared to control. There is supporting evidence that mitochondrial dysfunction in CHD reduces CL, a key phospholipid for oxidative phosphorylation (20, 21).
Our study demonstrated lower level of CL in CHD, especially TOF showed the lowest levels. NOX2 was also lower in CHD compared to control, revealing impaired ROS signaling (NOX2 role in embryonic heart development). Although there were no previous researches were done on the same marker in this particular sub group (TOF), but there is a similar study by Garcia et al. that reported total CL is significantly reduced in single ventricle heart failure, which may be secondary to elevated mitochondrial CL turnover and a compensatory response in the progression to HF (17). These findings are supported by Schlame and Greenberg,(22) who emphasized the essential role of CL in maintaining mitochondrial membrane architecture and function, chiefly in energy-demanding tissues like the heart.
Regarding genetic polymorphisms, we found that AC genotype of BMP4 (rs762642) SNP was dominant in CHD (83.3% vs. 10% in controls, p < 0.001), C allele frequency was higher in CHD (50% vs. 15%, p < 0.001).
Also, our study revealed that CT genotype carriers of ALCAT1 related to greater levels of CL and NOX2, compared to TT genotype among included patients.
Regarding combined biomarker model, we found that CL and NOX2 might improve risk stratification: low CL leads to mitochondrial dysfunction, low NOX2 causes impaired ROS signaling.
Logistic regression demonstrated CL as an independent predictor of CHD (OR = 1.006, p < 0.001), while NOX2 showed marginal significance (p = 0.100). Aligned with our findings, Kaemmerer et al. (23) demonstrated that multi-marker panels (e.g., CL + N -terminal-pro-brain natriuretic peptide) outperform single biomarkers in CHD prognosis.
There is contrasting evidence opposing ROS effects. Some studies have reported higher NOX2 activity (not low) as a CHD risk factor. This discrepancy may reflect disease-stage differences (early vs. advanced CHD) (24).
In our cohort, CL and NOX2 levels were positively correlated (r = 0.665, p<0.001), underscoring their interconnected roles in mitochondrial function and oxidative signaling. CL levels also showed significant negative correlations with age, operation time, blood loss, ICU stay, and FS, suggesting links with perioperative stress and surgical complexity.
Aligning with our results, Garcia et al. (17) found that CL content is significantly reduced in myocardial tissue from children with failing single-ventricle CHD, indicating mitochondrial lipid alterations in severe CHD phenotypes.
Although direct clinical studies linking NOX2 to CHD are limited, NOX2’s fundamental role in reactive oxygen species–mediated developmental signaling is supported in the broader cardiovascular literature. NOX2-generated ROS have been shown to participate in cardiac morphogenesis through EndMT, a process essential for valve and septal formation (25).
Our results revealed that reduced CL and NOX2 levels were significantly related to elevated risk of CHD, while specific polymorphisms in BMP4 (AC genotype), LCLAT1 (TT), and CYBB (CT) also correlated with altered CHD risk.
These findings are in agreement with Garcia et al. (17), who noted reduced CL levels in CHD patients, implicating mitochondrial dysfunction.
These observations align with known mitochondrial dysfunction in CHD, CL depletion compromises bioenergetics and respiratory chain integrity (26). In addition, BMP4 is essential for cardiac morphogenesis, and SNP rs762642 has been linked to increased CHD susceptibility in human cohorts (27).
Study limitations
The study has a relatively small sample size, which may limit the generalizability of findings, particularly for rare or complex CHD subtypes. Larger multicenter studies are needed to validate observed associations, especially for genetic polymorphisms. Lack of long- term follow up for associated morbidities and mortality, and multiple time assays of CL and NOX2 were also additional limitations
Conclusion
Our study concluded that reduced CL and NOX2 levels, together with specific genetic polymorphisms in BMP4, LCLAT1, and CYBB, are significantly related to the development of CHDs and may modulate postoperative prognosis. These biochemical and genetic markers appear to participate in the underlying pathogenic mechanisms of CHD, potentially through mitochondrial dysfunction, oxidative stress imbalance, and altered lipid remodeling. As such, they hold promise as predictive biomarkers for early screening, individualized risk stratification, and possibly targeted therapeutic interventions in affected pediatric populations.
Ethics: This study was approved by the Local Ethics Committee of the Faculty of Medicine, South Valley University (Approval Code: SVU-MED-MBC004-2-21-10-256). Written informed consent was obtained from all participants or their caregivers after a full explanation of the study's aims and procedures, in accordance with the Declaration of Helsinki
Peer-review: External and internal
Conflict of interest: None to declare
Authorship: M.H.H., A.T., A.F. , M.A., M.O., S.A.El-S., A. A.A., A.G., A.H., and M.F.A.H. equally contributed to preparation of manuscript and fulfilled authorship criteria
Acknowledgements and funding: None to declare
Statement on A.I.-assisted technologies use: AI-assisted technology was used for preparation of graphical abstract
Availability of data and materials: Contact corresponding author, data may be available upon reasonable request. In case of data sharing the fair use rules apply with acknowledgement of authors and source, or collaboration
References
| 1.Meng X, Song M, Zhang K, Lu W, Li Y, Zhang C, Zhang Y. Congenital heart disease: types, pathophysiology, diagnosis, and treatment options. MedComm 2024; 5: e631. doi: 10.1002/mco2.631 https://doi.org/10.1002/mco2.631 PMid:38974713 PMCid:PMC11224996 |
||||
| 2.Abdelbaseer KA, Abdelmawgood EA, Hassan MH, Ibrahem MH. Role of growth differentiation factor15 in pediatric cardiac patients, SVU-Int J Med Sci 2024; 7: 900-10. doi: 10.21608/svuijm.2022.164871.1416 https://doi.org/10.21608/svuijm.2022.164871.1416 |
||||
| 3.Dudek J, Hartmann M, Rehling P. The role of mitochondrial cardiolipin in heart function and its implication in cardiac disease. Biochim Biophys Acta Mol Basis Dis 2019; 1865: 810-21. doi: 10.1016/j.bbadis.2018.08.025 https://doi.org/10.1016/j.bbadis.2018.08.025 PMid:30837070 |
||||
| 4.Miao R, Wang L, Chen Z, Ge S, Li L, Zhang K, et al. Advances in the study of nicotinamide adenine dinucleotide phosphate oxidase in myocardial remodeling. Front Cardiovasc Med 2022; 9: 1000578. doi: 10.3389/fcvm.2022.1000578 https://doi.org/10.3389/fcvm.2022.1000578 PMid:36407440 PMCid:PMC9669076 |
||||
| 5.Ye D, Liu Y, Pan H, Feng Y, Lu X, Gan L, et al. Insights into bone morphogenetic proteins in cardiovascular diseases. Front Pharmacol 2023 ;14: 1125642. doi: 10.3389/fphar.2023.1125642 https://doi.org/10.3389/fphar.2023.1125642 PMid:36909186 PMCid:PMC9996008 |
||||
| 6.Wang Z, Liu XY, Yang CX, Zhou HM, Li YJ, Qiu XB, et al. Discovery and functional investigation of BMP4 as a new causative gene for human congenital heart disease. Am J Transl Res 2024; 16: 2034-48. doi: 10.62347/DGCD4269. https://doi.org/10.62347/DGCD4269 PMid:38883374 PMCid:PMC11170606 |
||||
| 7.Hao Y, Fan Y, Feng J, Zhu Z, Luo Z, Hu H, et al. ALCAT1-mediated abnormal cardiolipin remodelling promotes mitochondrial injury in podocytes in diabetic kidney disease. Cell Commun Signal 2024; 22: 26. doi: 10.1186/s12964-023-01399-4. https://doi.org/10.1186/s12964-023-01399-4 PMid:38200543 PMCid:PMC10777643 |
||||
| 8.Sumimoto H. Structure, regulation and evolution of Nox-family NADPH oxidases that produce reactive oxygen species. FEBS J 2008; 275: 3249-77. doi: 10.1111/j.1742-4658.2008.06488.x https://doi.org/10.1111/j.1742-4658.2008.06488.x PMid:18513324 |
||||
| 9.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007; 39: 175-91. doi: 10.3758/bf03193146 https://doi.org/10.3758/BF03193146 PMid:17695343 |
||||
| 10.Parinandi NL, Weis BK, Schmid HH. Assay of cardiolipin peroxidation by high-performance liquid chromatography. Chem Phys Lipids 1988; 49: 215-20. doi: 10.1016/0009-3084(88)90009-6 https://doi.org/10.1016/0009-3084(88)90009-6 PMid:3240565 |
||||
| 11.Pierpont ME, Brueckner M, Chung WK, Garg V, Lacro RV, McGuire AL, et al; American Heart Association Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; and Council on Genomic and Precision Medicine. Genetic basis for congenital heart disease: Revisited: A Scientific Statement From the American Heart Association. Circulation 2018; 138: e653-e711. doi: 10.1161/CIR.0000000000000606 https://doi.org/10.1161/CIR.0000000000000606 PMid:30571578 PMCid:PMC6555769 |
||||
| 12.Sparagna GC, Chicco AJ, Murphy RC, Bristow MR, Johnson CA, Rees ML, et al.. Loss of cardiac tetralinoleoyl cardiolipin in human and experimental heart failure. J Lipid Res 2007; 48: 1559-70. doi: 10.1194/jlr.M600551-JLR200 https://doi.org/10.1194/jlr.M600551-JLR200 PMid:17426348 |
||||
| 13.Massaro AN, El-Dib M, Glass P, Aly H. Factors associated with adverse neurodevelopmental outcomes in infants with congenital heart disease. Brain Dev 2008; 30: 437-46. doi: 10.1016/j.braindev.2007.12.013 https://doi.org/10.1016/j.braindev.2007.12.013 PMid:18249516 |
||||
| 14.Jamei Khosroshahi A, Shoaran M, Ghaffari S, Shabanpour E, Seraj Ebrahimi P, Ansari A, Khosravi R, et al. Growth pattern of children with congenital heart disease before and after open heart surgery. Front Pediatr. 2025; 13: 1463998. doi: 10.3389/fped.2025.1463998 https://doi.org/10.3389/fped.2025.1463998 PMid:40777158 PMCid:PMC12328431 |
||||
| 15.Blue GM, Kirk EP, Giannoulatou E, Sholler GF, Dunwoodie SL, Harvey RP, et al. Advances in the genetics of congenital heart disease: A clinician's guide. J Am Coll Cardiol 2017; 69: 859-70. doi: 10.1016/j.jacc.2016.11.060 https://doi.org/10.1016/j.jacc.2016.11.060 PMid:28209227 |
||||
| 16.Pasquali SK, He X, Jacobs JP, Jacobs ML, O'Brien SM, Gaynor JW. Evaluation of failure to rescue as a quality metric in pediatric heart surgery: an analysis of the STS Congenital Heart Surgery Database. Ann Thorac Surg 2012; 94: 573-9. doi: 10.1016/j.athoracsur.2012.03.065 https://doi.org/10.1016/j.athoracsur.2012.03.065 PMid:22633496 PMCid:PMC3828205 |
||||
| 17.Garcia AM, McPhaul JC, Sparagna GC, Jeffrey DA, Jonscher R, Patel SS, Sucharov CC, Stauffer BL, Miyamoto SD, Chatfield KC. Alteration of cardiolipin biosynthesis and remodeling in single right ventricle congenital heart disease. Am J Physiol Heart Circ Physiol. 2020;318(4):H787-H800. doi: 10.1152/ajpheart.00494.2019. https://doi.org/10.1152/ajpheart.00494.2019 PMid:32056460 PMCid:PMC7191493 |
||||
| 18.Moazzen H, Wu Y, Engineer A, Lu X, Aulakh S, Feng Q. NOX2 Is critical to endocardial to mesenchymal transition and heart development. Oxid Med Cell Longev 2020; 2020: 1679045. doi: 10.1155/2020/1679045 https://doi.org/10.1155/2020/1679045 PMid:32655758 PMCid:PMC7320281 |
||||
| 19.Vanreusel I, Taeymans J, Van Craenenbroeck E, Segers VFM, Van Berendoncks A, Briedé JJ, et al. Elevated oxidative stress in patients with congenital heart disease and the effect of cyanosis: a meta-analysis. Free Radic Res 2023; 57: 470-86. doi: 10.1080/10715762.2023.2284639 https://doi.org/10.1080/10715762.2023.2284639 PMid:38000042 |
||||
| 20.Zhang H, Yu F, Tian Z, Jia D. Cardiolipin remodeling in cardiovascular diseases: implication for mitochondrial dysfunction. Acta Physiol (Oxf) 2025; 241: e70073. doi: 10.1111/apha.70073 https://doi.org/10.1111/apha.70073 PMid:40530586 |
||||
| 21.Jia D, Zhang J, Nie J, Andersen JP, Rendon S, Zheng Y, et al. Cardiolipin remodeling by ALCAT1 links hypoxia to coronary artery disease by promoting mitochondrial dysfunction. Mol Ther 2021; 29: 3498-511. doi: 10.1016/j.ymthe.2021.06.007 https://doi.org/10.1016/j.ymthe.2021.06.007 PMid:34111561 PMCid:PMC8636157 |
||||
| 22.Schlame M, Greenberg ML. Biosynthesis, remodeling and turnover of mitochondrial cardiolipin. Biochim Biophys Acta Mol Cell Biol Lipids 2017; 1862: 3-7. doi: 10.1016/j.bbalip.2016.08.010 https://doi.org/10.1016/j.bbalip.2016.08.010 PMid:27556952 PMCid:PMC5125896 |
||||
| 23.Kaemmerer H, Fratz S, Braun SL, Koelling K, Eicken A, Brodherr-Heberlein S, et al. Erythrocyte indexes, iron metabolism, and hyperhomocysteinemia in adults with cyanotic congenital cardiac disease. Am J Cardiol 2004; 94: 825-8. doi: 10.1016/j.amjcard.2004.06.014 https://doi.org/10.1016/j.amjcard.2004.06.014 PMid:15374802 |
||||
| 24.Sirker A, Zhang M, Shah AM. NADPH oxidases in cardiovascular disease: insights from in vivo models and clinical studies. Basic Res Cardiol 2011; 106: 735-47. doi: 10.1007/s00395-011-0190-z https://doi.org/10.1007/s00395-011-0190-z PMid:21598086 PMCid:PMC3149671 |
||||
| 25.Nocella C, D'Amico A, Cammisotto V, Bartimoccia S, Castellani V, Loffredo L, et al. Structure, activation, and regulation of NOX2: At the crossroad between the innate immunity and oxidative stress-mediated pathologies. Antioxidants (Basel) 2023; 12: 429. doi: 10.3390/antiox12020429 https://doi.org/10.3390/antiox12020429 PMid:36829988 PMCid:PMC9952346 |
||||
| 26.Paradies G, Paradies V, Ruggiero FM, Petrosillo G. Role of cardiolipin in mitochondrial function and dynamics in health and disease: molecular and pharmacological aspects. Cells 2019; 8: 728. doi: 10.3390/cells8070728 https://doi.org/10.3390/cells8070728 PMid:31315173 PMCid:PMC6678812 |
||||
| 27.Qian B, Mo R, Da M, Peng W, Hu Y, Mo X. Common variations in BMP4 confer genetic susceptibility to sporadic congenital heart disease in a Han Chinese population. Pediatr Cardiol 2014; 35: 1442-7. doi: 10.1007/s00246-014-0951-1 https://doi.org/10.1007/s00246-014-0951-1 PMid:25022354 PMCid:PMC4236636 |
||||
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

