AVEIR leadless pacemaker implantation in a patient with a small right ventricle
CASE REPORT
AVEIR leadless pacemaker implantation in a patient with a small right ventricle
Article Summary
- DOI: 10.24969/hvt.2025.601
- CARDIOVASCULAR DISEASES
- Published: 19/10/2025
- Received: 25/08/2025
- Revised: 05/10/2025
- Accepted: 05/10/2025
- Views: 1843
- Downloads: 753
- Keywords:
Address for Correspondence: Gaziza Marat, Heart Rhythm Scientific Research Institute, NpJSC 'Astana Medical University, Astana, Kazakhstan
Email: g.marat87@gmail.com
ORCID: Ayan S. Abdrakhmanov 0000-0001-6315-5016; Gaziza Marat 0009-0005-4947-1940; Zhanasyl A. Suleymen 0009-0008-3262-4551
Facebook: facebook.com/ayan abdrakhmanov; facebook.com/gaziza.ismagulova; facebook.com/zhanasyl suleymen
Authors: Ayan S. Abdrakhmanov¹,², Gaziza Marat¹*, Zhanasyl A. Suleymen¹
¹Heart Rhythm Scientific Research Institute, NpJSC 'Astana Medical University', Astana, Kazakhstan
²Heart Rhythm Center, 'National Coordination Center for Emergency Medicine' of the Ministry of Healthcare of the Republic of Kazakhstan, Astana, Kazakhstan
Abstract
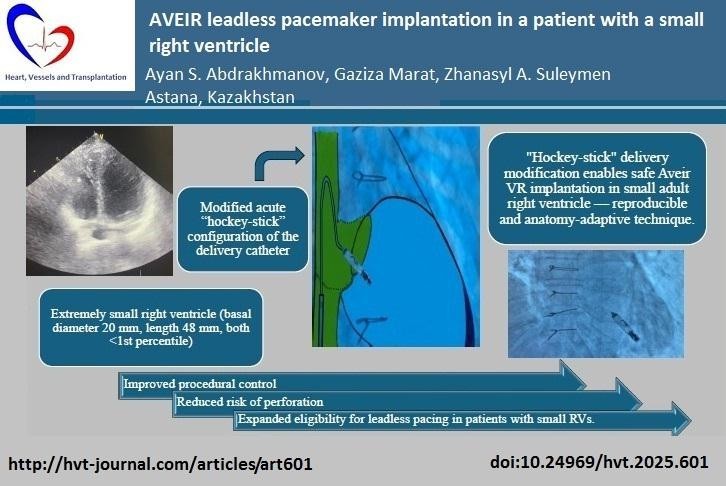
Objective: To describe a reproducible “hockey-stick” modification of the Aveir VR (Abbott, USA) delivery technique enabling safe leadless pacing in a patient with an exceptionally small adult right ventricle.
Case presentation: A 64-year-old woman with sick sinus syndrome, tachycardia-bradycardia syndrome, and intermittent Mobitz II AV block presented with symptoms requiring permanent pacing. Echocardiography revealed an extremely small right ventricle (RV) (basal diameter 20 mm, length 48 mm, both <1st percentile). Conventional transvenous pacing was considered high-risk due to the patient’s exceptionally small right ventricle. The challenge was not related to the final positioning of the device within the RV, but rather to the passage of the delivery system across the tricuspid valve. Because the Aveir VR (Abbott, USA) has a length of 38 mm, in asthenic patients with a “drop-like heart” the catheter, when introduced from the inferior approach, abuts the anterior RV wall and creates an acute angle, making safe advancement difficult. To overcome this, our team developed and applied for the first time a modified acute “hockey- stick configuration of the delivery catheter. By introducing additional curvature from the superior vena cava, with this newly developed and first-time applied technique by our team, we successfully overcame the restricted ventricular cavity and implanted the device in the apical-septal region without complications. Importantly, no description of such a technique has been identified in the available literature. Follow-up at 6 and 12 months confirmed stable electrical parameters and sustained symptomatic improvement.
Conclusion: In anatomically constrained ventricles, a controlled, mapping-first, helix-fixation approach with an acute delivery curve may expand candidacy for leadless pacing while maintaining safety.
Key words: Small right ventricle, leadless pacemaker implantation, pacing
Introduction
Leadless pacing has transformed the management of bradyarrhythmias by eliminating complications associated with leads and device pockets (1, 2). However, unique anatomical challenges, such as an extremely small right ventricle (RV), pose technical difficulties that can limit safe implantation (3, 4). Current evidence supports the safety of both Micra™ (Medtronic, USA) and Aveir™ VR systems (Abbott, USA), yet procedural adaptation may be required in patients with challenging anatomy (1, 2).
We report the first case of Aveir VR (Abbott, USA) implantation in a patient with a markedly diminutive RV, achieved through an innovative “hockey-stick” delivery modification.
Case Presentation
A 64-year-old woman with a history of redo mitral valve replacement with a bioprosthesis in 2020 (following prior mechanical valve replacement), ischemic stroke, and infective endocarditis was admitted with complaints of recurrent dizziness, syncope, and palpitations. She had an asthenic body habitus (body mass index 18.1 kg/m²).
Graphical abstract
Baseline ECG (Fig. 1) showed sinus bradycardia with a prolonged PQ interval. Holter monitoring (Fig.2) revealed intermittent second-degree atrioventricular block (Mobitz II) with a maximum pause of 2154 ms.
In addition, episodes of tachycardia-bradycardia syndrome with periods of QT interval prolongation were observed.
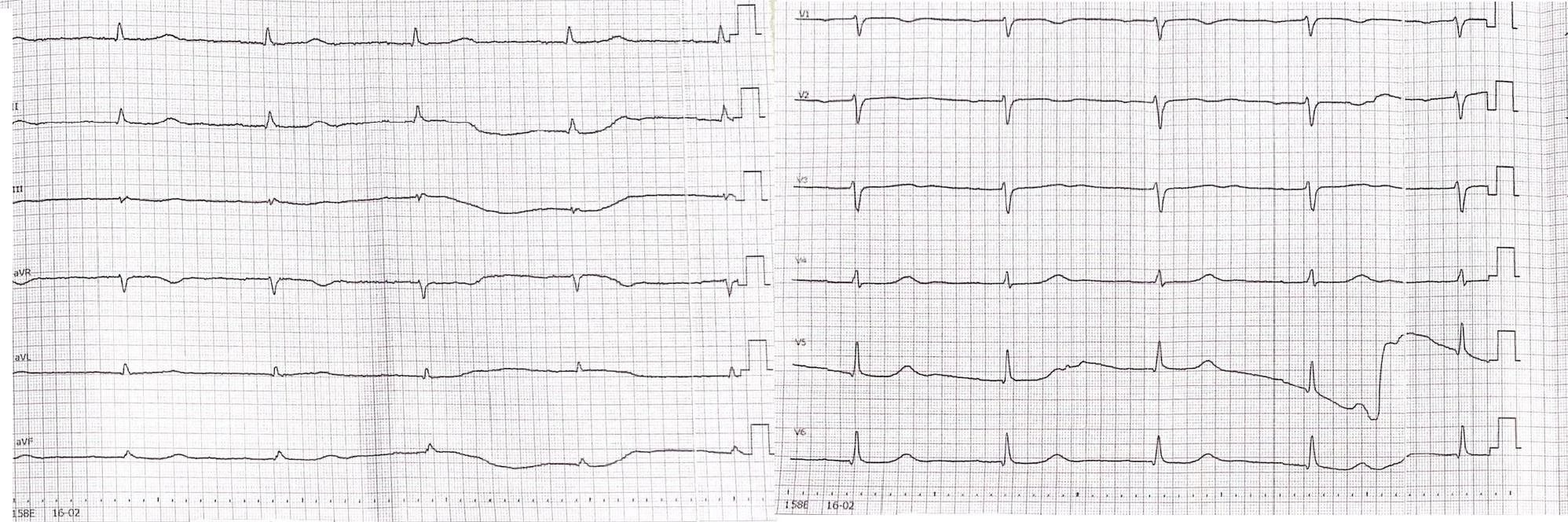
Figure 1. Electrocardiogram (ECG) tracing demonstrating sinus bradycardia and a prolonged PQ interval
10 mm/mV, paper speed 50 mm/sec
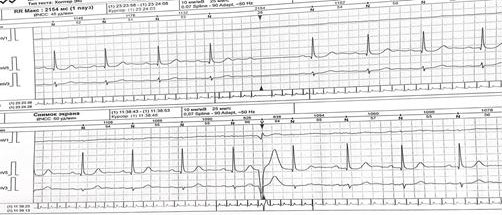
Figure 2. Holter monitor strip revealing an episode of second-degree atrioventricular block (Mobitz II) with a 2154 ms pause
Transthoracic echocardiography (Fig. 3) confirmed preserved left ventricular systolic function ( left ventricular ejection fraction 58%) but demonstrated a markedly small RV, measuring only 20 mm in basal diameter and 48 mm in longitudinal length. According to ASE/EACVI reference values, the normal basal RV diameter in adults is 25–41 mm (mean 33 (4) mm) and the longitudinal length is 59–83 mm (mean 71 (6) mm). In our patient, the z-scores for basal diameter and length were −3.25 and −3.83, respectively, corresponding to below the 1st percentile. These findings confirm an exceptionally small RV creating unique technical challenge for device implantation.
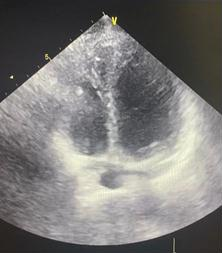
Figure 3. Transthoracic echocardiographic image demonstrating a small right ventricular cavity
Under fluoroscopic guidance, the delivery catheter was advanced through the right atrium. Significant resistance was encountered as the catheter tip repeatedly abutted the ventricular wall due to the limited chamber size. To overcome this, the catheter was reshaped into a “hockey-stick” configuration developed by our team (Fig. 4–9). This modification enabled safe passage through the tricuspid valve and facilitated navigation to the apical-septal region. Contact mapping confirmed optimal electrical parameters before fixation, and the device was screwed into the septum. Device interrogation demonstrated proper function: pacing threshold 0.75 V at 0.4 ms, R- wave amplitude 6.0 mV, impedance 750 Ω, mode VVI, with estimated battery longevity of ~12 years.
Periprocedural anticoagulation was managed with dabigatran 150 mg twice daily; the morning dose on the day of procedure was withheld and resumed 12 hours post-implantation. The patient was discharged home in stable condition without complications, with continued anticoagulation due to the presence of a bioprosthetic mitral valve.
Methods (Technical description)
Implantation (Fig.4-9) was performed via the right femoral vein under fluoroscopic guidance using the Aveir™ VR leadless pacemaker system (Abbott, USA). After ultrasound-guided venous access, a 0.035ʺ guidewire was advanced to the superior vena cava, and a 25F/27F introducer sheath was inserted.
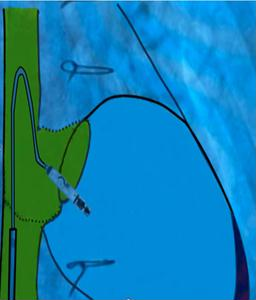
Figure 4. Modified 'hockey-stick' curvature of the delivery catheter guiding the pacemaker toward the right ventricle
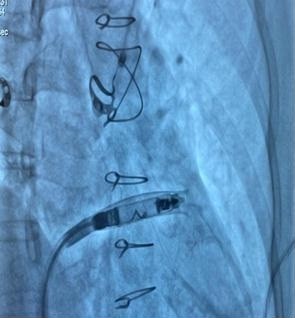
Figure 5. Implantation phase of the pacemaker
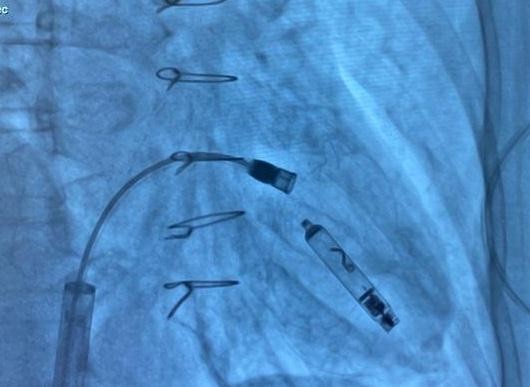
Figure 6. Fluoroscopic image showing the Aveir™ VR leadless pacemaker positioned in the right ventricular septum
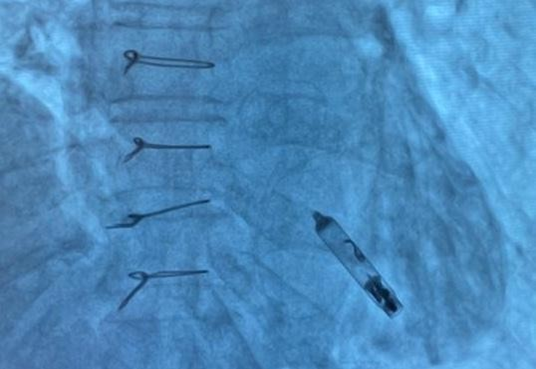
Figure 7. Final position of the leadless pacemaker inside the small right ventricle
At the junction of the superior vena cava and right atrium, maximal deflection of the distal catheter segment was applied, followed by slight retraction, creating an acute 70–90° bend resembling a “hockey stick.” This configuration shortened the working length, allowing maneuverability in the restricted RV cavity. Crossing the tricuspid valve was guided by RAO 30° and LAO 40° projections to ensure septal alignment. In the apical-septal region, contact mapping (with the helix unreleased) confirmed acceptable parameters (R-wave ≥5 mV, threshold ≤1.0 V at 0.4 ms, impedance 400– 1500 Ω). Upon satisfactory values, the device was screwed into the septum with 1.5 turns. Perpendicular positioning was confirmed in orthogonal views, followed by a tug test. Repositioning was feasible if parameters were suboptimal.
The delivery system was then withdrawn, and hemostasis achieved with vascular closure.
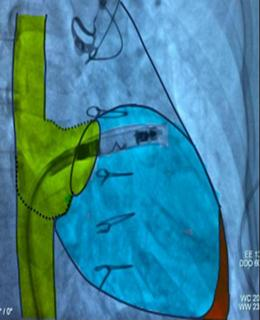
Figure 8. Schematic representation of the catheter- based approach
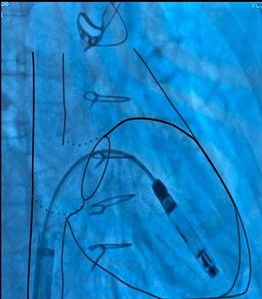
Figure 9. Schematic overlay showing the optimal angle
of delivery for device implantation in an anatomically restricted right ventricle
Results
Immediate device interrogation confirmed stable electrical performance: pacing threshold 0.75 V at 0.4 ms, R-wave 6.0 mV, impedance 750 Ω, estimated longevity ~12 years. At 6 months, follow-up showed: R- wave 6.2 mV, threshold 0.75 V, impedance 720 Ω, projected longevity 12 years. No complications were
observed, and the patient remained asymptomatic. At 12 months, values remained stable: R-wave 6.0 mV, threshold 0.80 V, impedance 680 Ω, projected longevity 20 years. No hospitalizations were required. Table 1 summarizes the intraprocedural and follow-up electrical parameters.
|
Table 1. Electrical parameters and clinical outcomes at implantation, 6 and 12 months |
|||
|
Parameter |
Intraoperative |
6 months |
12 months |
|
R-wave amplitude, mV |
6.0 |
6.2 |
6.0 |
|
Pacing threshold, V at 0.4 ms |
0.75 |
0.75 |
0.80 |
|
Impedance, Ω |
750 |
720 |
680 |
|
Mode |
VVI |
VVI |
VVI |
|
Battery longevity, years |
~12 |
20 |
20 |
Discussion
Leadless pacing offers substantial advantages over conventional systems by avoiding leads and device pockets. In patients with extremely small RVs, however, safe implantation may be technically challenging. Our case demonstrates a novel “hockey-stick” modification of the Aveir VR delivery system that enabled successful implantation in a ventricle measuring far below normal adult ranges. The decision to adopt a ‘hockey-stick’ modification was driven by the extremely small RV cavity, where a standard curvature repeatedly abutted the ventricular wall, preventing access to the apical- septal region and increasing the risk of perforation.
To address this challenge, our team developed and applied for the first time a novel acute ‘hockey-stick’ curvature of the delivery catheter, which reduced the effective working length and enabled precise septal alignment.
In the context of complex RV anatomy, the literature confirms the feasibility of leadless pacing when implantation strategy is individualized and preferentially targets the interventricular septum. In cohorts with congenital heart disease, pediatric patients, and those with diminutive RVs, success has been associated with septal positioning rather than free wall placement, pre- procedural imaging with echocardiography/computed tomography, and active curve control of the delivery system. Comparing platforms, systems with helix fixation Aveir VR (Abbott, USA) offer advantages in constrained RVs: pre-fixation contact mapping, controlled repositioning, and the option for dual- chamber upgrade Aveir DR (Abbott, USA). These features may be decisive when optimal parameters and secure fixation are required, whereas tine-fixation systems Micra (Medtronic, USA) allow parameter assessment only after deployment (5).
Why Aveir (Abbott, USA) and not Micra (Medtronic, USA)?
While Micra™ leadless pacing (Medtronic, USA) was also a theoretical option, its tine-based fixation allows assessment of electrical parameters only after deployment. In a markedly small RV, this may increase the risk of unstable thresholds or perforation. By contrast, the Aveir VR system (Abbott, USA) provides helix-based fixation with pre-fixation mapping, controlled repositioning, and the potential for dual- chamber upgrade Aveir DR (Abbott, USA). These features made Aveir VR (Abbott, USA) a safer and more appropriate choice in our patient with a diminutive RV and binodal disease.
Potential complications and management
In patients with small right ventricles, reported risks include perforation, cranial migration into the RV outflow tract, and unstable pacing thresholds. Risk mitigation strategies involve strict septal targeting, limited forward force during catheter manipulation, orthogonal projection verification (RAO/LAO), and performance of a tug test to ensure secure fixation. If electrical parameters are suboptimal, immediate unscrewing and repositioning are recommended (1, 6). In cases of suspected perforation or tamponade, urgent echocardiography, pericardiocentesis, and surgical backup are essential. Device dislodgement may be managed by repositioning or, in rare cases, retrieval via the delivery system.
Learning curve and operator experience
Experience from early adopting centers suggests that the Aveir VR (Abbott, USA) implantation technique has a relatively short learning curve. Operators typically achieve procedural proficiency after only a few implantations, which make the method reproducible outside of highly specialized centers (1-3). Nevertheless, successful implantation in anatomically challenging cases, such as in patients with a diminutive right ventricle, requires careful fluoroscopic visualization in orthogonal projections and familiarity with septal targeting. Thus, while the implantation technique itself can be mastered relatively quickly, optimal outcomes depend on operator experience with advanced navigation and mapping skills.
Cost-effectiveness
Although leadless pacemakers such as Aveir VR (Abbott, USA) are associated with higher upfront device costs compared with conventional transvenous systems, cost- effectiveness analyses have suggested potential long- term benefits due to reduced risks of pocket- and lead- related complications, shorter hospitalization, and fewer reinterventions (7). In anatomically challenging cases, where conventional pacing may be unsafe or unfeasible, the economic argument is outweighed by the clinical necessity.
Take-home message
Even in patients with extremely small right ventricles, leadless pacemaker implantation can be safely accomplished. A “hockey-stick” catheter modification provides secure navigation, optimal septal positioning, and stable long-term outcomes, expanding the role of leadless pacing in anatomically constrained populations.
Conclusion
This is the first reported case of Aveir VR (Abbott, USA)
implantation in an adult with a markedly small right ventricle using a modified “hockey-stick” delivery approach. The case highlights the adaptability of leadless pacing technology and the value of individualized procedural strategies in overcoming anatomical limitations.
Ethics: The procedure was performed in accordance with institutional policies and the principles of the Declaration of Helsinki 2024. The case report was approved by the local ethics committee, and written informed consent was obtained from the patient for both the procedure and the publication of anonymized clinical data and images.
Conflict of interest: None to declare
Peer-review: External and internal Authorship: A.S.A., G.M. and Zh.A.S. equally contributed to case management, manuscript preparation and fulfilled the authorship criteria
Acknowledgements and funding: None to declare
Statement on A.I.-assisted technologies use: Authors declare they did not use A.I. assisted technologies in
preparation of manuscript
Data and material availability: Does not apply
References
| 1. Ngo L, Nour D, Denman RA, Walters TE, Haqqani HM, Woodman RJ, et al. Safety and efficacy of leadless pacemakers: A systematic review and meta-analysis. J Am Heart Assoc 2021;10: e019212. DOI: 10.1161/JAHA.120.019212 https://doi.org/10.1161/JAHA.120.019212 PMid:34169736 PMCid:PMC8403316 |
||||
| 2. El-Chami MF, Johansen JB, Zaidi A, Clementy N, Garweg C, Mijic D, et al. Safety and efficacy of the Aveir leadless pacemaker: 6-month results from the LEADLESS II IDE study. JACC Clin Electrophysiol 2023; 9: 23-31. DOI: 10.1016/j.jacep.2022.08.015 https://doi.org/10.1016/j.jacep.2022.08.015 PMid:36424003 |
||||
| 3.Knops RE, Tjong FVY, Neuzil P, Johansen JB, Beauregard LA, van Gelder IC, et al. Leadless pacemaker implantations: Procedural characteristics in a real-world setting. Europace2021; 23: 588-95. DOI: 10.1093/europace/euaa311 https://doi.org/10.1093/europace/euaa311 PMid:33279992 |
||||
| 4.Das S, Boe BA, Saef J, Chan KC, Kilinc O, Bibevski S, Roth TS. Leadless pacemaker implantation in Fontan patients with multimodality imaging: tips and tricks. J Innov Card Rhythm Manag 2024; 15: 5990-6. doi:10.19102/icrm.2024.15082 https://doi.org/10.19102/icrm.2024.15082 PMid:39193536 PMCid:PMC11346501 |
||||
| 5.Tam MTK, Cheng YW, Chan JYS, Chan CP, Au ACK, Fan KWS, et al. Aveir VR real-world performance and chronic pacing threshold prediction using mapping and fixation electrical data. Europace 2024; 26: euae051. doi:10.1093/europace/euae051 https://doi.org/10.1093/europace/euae051 PMid:38457487 PMCid:PMC10923508 |
||||
| 6.Shah MJ, Borquez AA, Cortez D, McCanta AC, De Filippo P, Whitehill RD, et al. Transcatheter leadless pacing in children: a PACES collaborative study in the real-world setting. Circ Arrhythm Electrophysiol 2023; 16: e011447. doi:10.1161/CIRCEP.122.011447 https://doi.org/10.1161/CIRCEP.122.011447 |
||||
| 7.Lago-Quinteiro JR, Reyes-Santias F, Antelo M, Caballer-Tarazona V, Martinez-Sande JL, Garcia-Seara J, et al. Single-chamber pacemakers: with or without leads? Cost-effectiveness and cost-utility analyses. Ann Med 2025; 57: 2512108. | ||||
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

