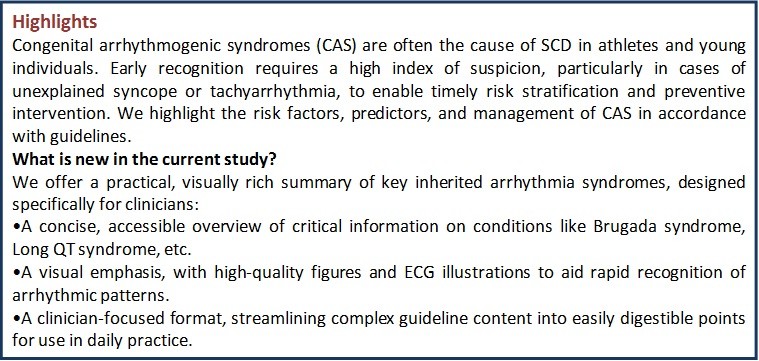
Sudden cardiac death in congenital arrhythmogenic syndromes: Risk stratification and management
REVIEW
Sudden cardiac death in congenital arrhythmogenic syndromes: Risk stratification and management
Article Summary
- DOI: 10.24969/hvt.2025.602
- CARDIOVASCULAR DISEASES
- Published: 26/10/2025
- Received: 24/09/2025
- Revised: 12/10/2025
- Accepted: 12/10/2025
- Views: 1954
- Downloads: 1169
- Keywords: Anti-arrhythmic drugs, Early repolarization syndrome, Long QT syndrome, Sudden cardiac death, Brugada syndrome
Address for Correspondence: Samir Morcos Rafla, Cardiology Department , Faculty of Medicine, Alexandria University, Alexandria, Egypt
E-mail: smrafla1@gmail.com Mobile (Whatsapp): +201001495577
ORCID: Samir Morcos Rafla - 0000-0001-8688-6532- Moustafa Eissa Nawar 0000-0002-0281-9269; Mohamed Ibrahim Sanhoury 0000-0002-6563-0607; 0000-0002-6563-0607; Moustafa Ali Dawood - 0000-0001-8509-5982; Ashraf Abdelaziz Elamin - 0000-0002-7784-1606; Aly Aboelhoda Ahmed - 0000-0002-9557-9873; Moataz Ahmed Zaki 0000-0002-9390-2681; Hany Samir Assaad - 0000-0002-1501-9033
Twitter (X): Samir Morcos Rafla - Twitter @rafla_samir Facebook: Samir Morcos Rafla – Samir Rafla
Samir Morcos Rafla1a*, Moustafa Eissa Nawar1a, Mohamed Ibrahim Sanhoury1a, Moustafa Ali Dawood1a, Ashraf Abdelaziz Elamin1a, Aly Aboelhoda Ahmed1a, Moataz Ahmed Zaki2, Hany Samir Assaad1b
1aCardiology Department and 1bCritical Care Department, Faculty of Medicine, Alexandria University, Alexandria, Egypt
2Medical Research Institute, Alexandria University, Alexandria, Egypt
Abstract
In young individuals, sudden cardiac death (SCD) is often caused by genetic heart diseases, which are usually classified into inherited cardiomyopathies and primary electrical heart diseases.
The aim of the review is to describe primary electrical diseases and define predictors of SCD. Primary electrical diseases (more commonly) encountered in clinical practice including Brugada syndrome (BrS) and long QT syndrome (LQTS). Several risk prediction tables have been developed to estimate the likelihood of SCD after identifying multiple risk factors. Syndromes that need ICD, cardiac arrest survivors, arrhythmogenic right ventricular dysplasia, LQTS or BrS with syncope or + need for electrophysiological study, early repolarization syndrome, Wolff-Parkinson-White Syndrome with ventricular tachycardia, catecholaminergic polymorphic ventricular tachycardia.
Congenital arrhythmogenic syndromes are relatively common and represent an important cause of SCD in athletes and young individuals. Early recognition requires a high index of suspicion, particularly in cases of unexplained syncope or tachyarrhythmia, to enable timely risk stratification and preventive intervention.
Key words: Anti-arrhythmic drugs, early repolarization syndrome, long QT syndrome, sudden cardiac death, Brugada syndrome
![]() Graphical abstract
Graphical abstract
Introduction
In young individuals, sudden cardiac death (SCD) is often caused by genetic heart diseases, which are usually classified into inherited cardiomyopathies and primary electrical heart diseases.
The aim of the review is to describe primary electrical diseases and define predictors of SCD. Primary electrical diseases include: arrhythmogenic right ventricular cardiomyopathy (ARVC), early repolarization syndrome (ERS), Brugada syndrome (BrS), Long QT Syndrome (LQTS), Wolff-Parkinson-White syndrome (WPW) with hemodynamically unstable ventricular tachycardia (VT), and catecholaminergic polymorphic ventricular tachycardia (CPVT) (1-4). Other syndromes not included in this paper are: hypertrophic cardiomyopathy (HCM), short QT syndrome, congenital complete heart block, and idiopathic ventricular tachyarrhythmias.
Arrhythmogenic right ventricular cardiomyopathy (ARVC)
ARVC is a hereditary disease characterized by fibrofatty replacement of the right ventricular (RV) myocardium, increasing the risk of ventricular arrhythmias and sudden cardiac death (SCD) (5-10). Several key risk indicators have been identified (5, 6, 8, 11).
Diagnostic Criteria
Imaging (echocardiography, computed tomography, CT and magnetic resonance imaging, MRI) reveals structural and/or functional abnormalities of the right ventricle (RV) (5, 8):
RV Dilatation: enlargement of the RV outflow tract (RVOT) in parasternal short- and long-axis views, indexed RV end-diastolic area > 110 mm²/m² suggests dilatation.
Regional wall motion abnormalities of RV: akinesia, dyskinesia, or aneurysmal bulging, often in the “triangle of dysplasia”: RV inflow, RV outflow tract (RVOT), apex.
Reduced RV fractional area change (FAC): FAC < 33% indicates systolic dysfunction.
RVOT diameter: 32 mm (parasternal long-axis) or > 36 mm (short-axis) are abnormal.
B. Right ventricular function: TAPSE (Tricuspid Annular Plane Systolic Excursion) - decreased (<16 mm) reflects RV systolic impairment. Tissue Doppler S′ velocity (lateral tricuspid annulus) - < 10 cm/s is suggestive of dysfunction.
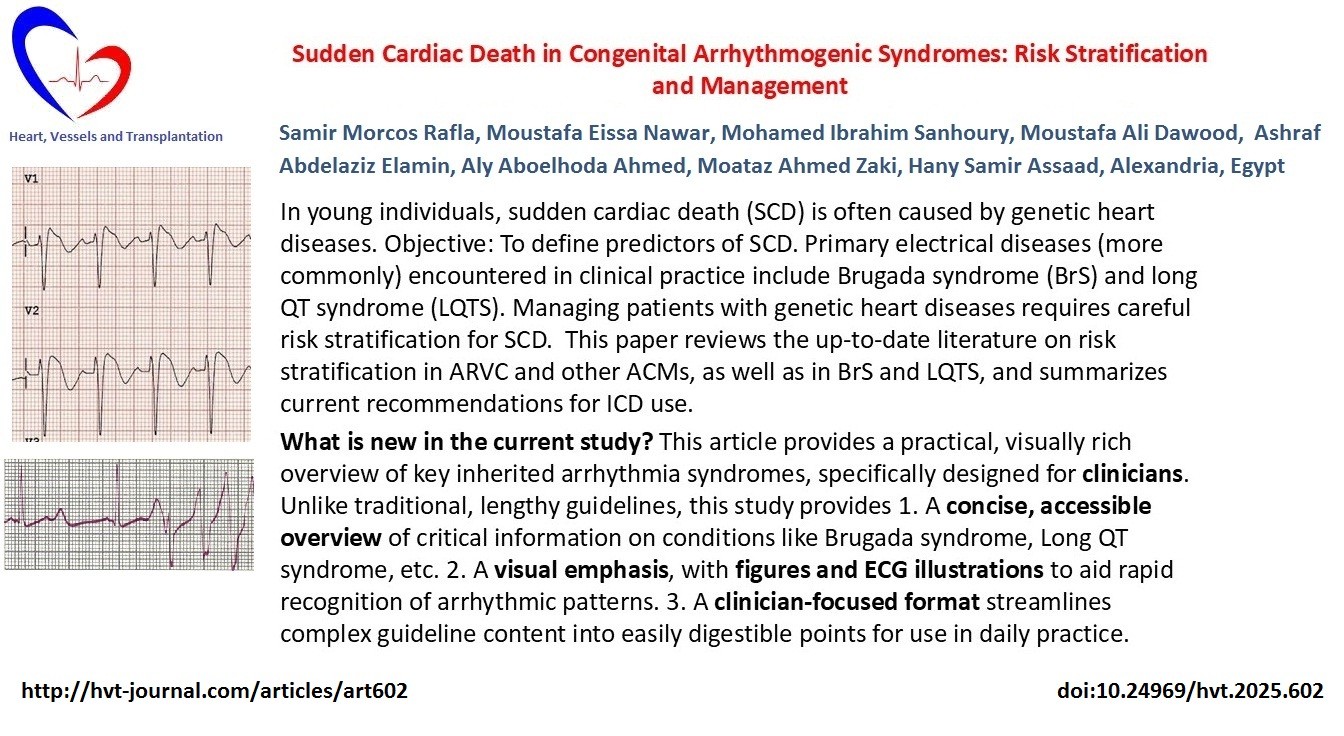
Electrocardiogram (ECG) (Fig. 1): Often shows right bundle branch block (RBBB). Updated criteria emphasize left ventricular (LV) depolarization/repolarization abnormalities and arrhythmias originating from the LV (8, 9).
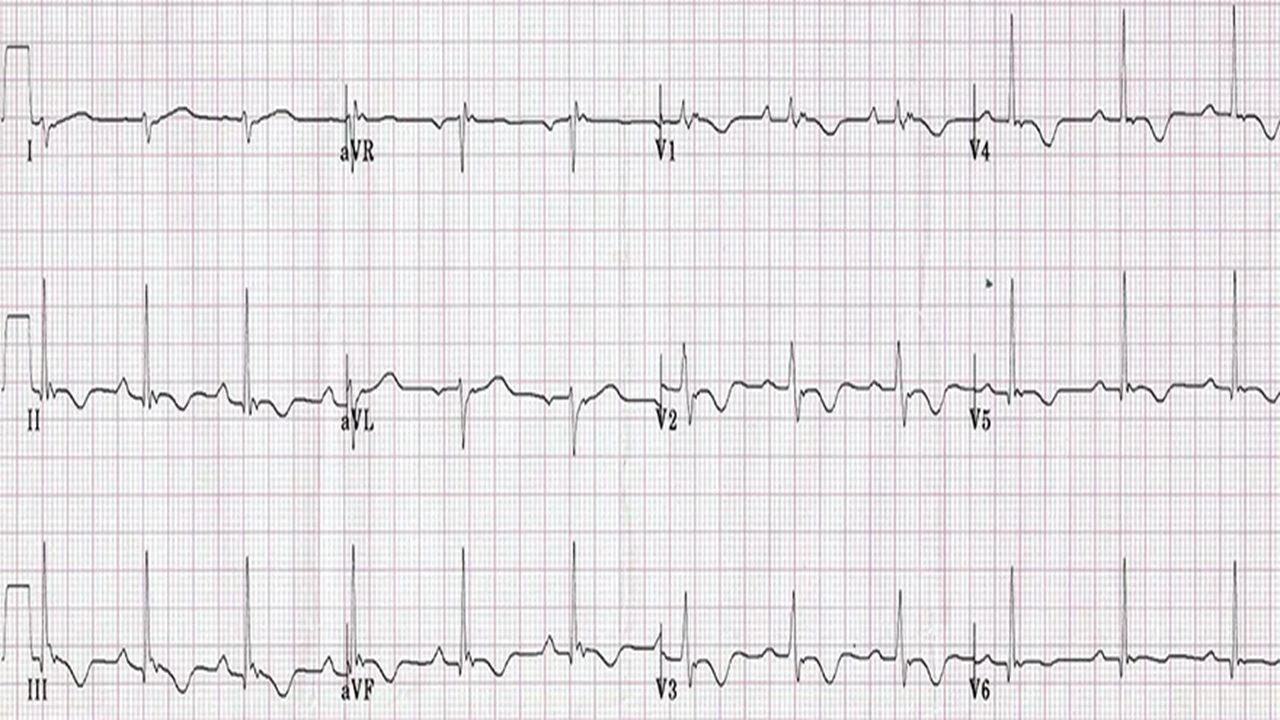
Figure 1. Arrhythmogenic right ventricular dysplasia. Note the Epsilon wave in V1, and T inversion in many leads.
Signal-averaged ECG: May show late potentials, supporting the diagnosis (8).
Late gadolinium enhancement (LGE) on MRI: Detects myocardial scarring. A "ring-like" LGE pattern suggests arrhythmogenic left ventricular cardiomyopathy (ALVC) (8, 11).
Risk of sudden cardiac death (SCD)
Major risk predictors (5, 6, 8, 11-15):
• Prior cardiac arrest or documented ventricular fibrillation (VF)
• Unexplained syncope
• Sustained or non-sustained VT (especially sustained)
• Severe RV dysfunction
• Male sex and younger age at diagnosis
• Symptomatic patients with definite ARVC, moderate RV/LV dysfunction, non-sustained VT (NSVT), or inducible sustained monomorphic VT during programmed electrical stimulation (PES)
• T-wave inversion in multiple leads (8, 9)
• High burden of premature ventricular contractions (PVCs) (5, 6)
Management of ARVC
Lifestyle: Avoid or limit strenuous physical activity (5, 6, 8).
Pharmacological therapy:
• Beta-blockers (first-line)
• Antiarrhythmic drugs (e.g., sotalol, amiodarone) in selected cases
• Standard heart failure therapy (including transplant in advanced cases) (5, 6, 11)
Device therapy (Implantable cardioverter-defibrillator, ICD): Indicated in patients with (5, 6, 11):
• Resuscitated cardiac arrest
• Hemodynamically unstable sustained VT
• Severe RV or LV systolic dysfunction
• Hemodynamically stable sustained VT (Class IIa)
• Syncope likely due to arrhythmia (Class IIa)
• Multiple risk factors (Class IIa/IIb)
Interventional therapy:
Catheter ablation targeting VT substrates may be considered. Recurrence is common and repeat procedures may be necessary (6, 11).
Guidelines
Class I Recommendations
• CMR (cardiac magnetic resonance): Recommended for patients with suspected ARVC (Class I) (11).
• ICD (implantable cardioverter-defibrillator): Recommended for patients with confirmed ARVC and arrhythmic syncope.
• Secondary prevention of SCD (Class I): ICD implantation is recommended for patients with documented VF or hemodynamically significant VT, in the absence of reversible causes (5, 6, 11) .
Early repolarization syndrome (ERS)
Unlike ARVC, which is a structural heart disease, ERS is a purely electrical abnormality identified by specific ECG patterns (8, 16-21). Once considered benign (18-20), ERS is now recognized as a potential cause of idiopathic VF and SCD—particularly in patients without structural heart disease (2, 19, 21-26, 27-29).
Major risk indicators for SCD in ERS
ECG features (20-24) (Fig. 2)
•J-point elevation >0.1 mV, especially >0.2 mV, is linked to increased arrhythmic risk.
•ST-segment morphology: Horizontal or descending ST segments are associated with higher risk.
•QRS complex: Notching or slurring at the J-point is considered pro-arrhythmic.
•J-wave dynamics: Longer duration and wider angle indicate a more malignant substrate.
•T/R wave ratio: A lower ratio suggests a more arrhythmogenic profile.
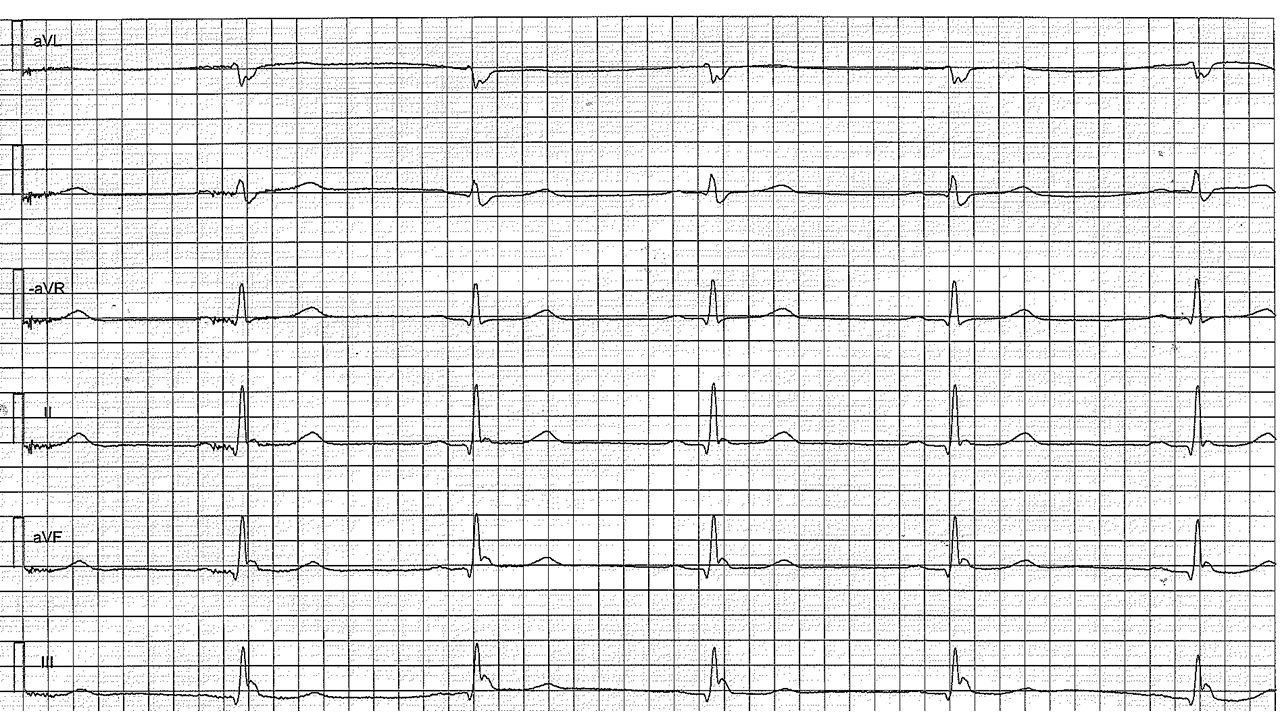
Figure 2. ECG showing features of ERS—J-point elevation, and ST-segment elevation in the inferior leads
ECG – electrocardiogram, ERS - early repolarization syndrome
Demographic factors (16-18, 23)
ERS is more common in:
•Males, particularly younger athletes
•Individuals of African descent
The risk of SCD is highest in middle-aged adults (23, 24).
Clinical history (8, 22–24)
•Unexplained syncope or resuscitated cardiac arrest significantly increases risk.
•A family history of SCD or arrhythmias in first-degree relatives indicates a possible genetic predisposition.
Coexisting cardiac conditions (16, 20, 24)
Although ERS usually occurs in structurally normal hearts, the presence of underlying cardiomyopathies or other heart diseases significantly elevates the risk of malignant arrhythmias.
Clinical Implications
ERS should be considered in:
•Patients with unexplained syncope
•Individuals with a history of sudden cardiac arrest, even in the absence of structural abnormalities (8, 19, 21-24).
Risk stratification requires:
•Detailed ECG analysis
•Assessment of symptoms
•Thorough family history evaluation
In high-risk individuals, EPS may guide management decisions (21-24).
ICD implantation may be considered for patients with high-risk ERS patterns, but treatment should be individualized (22–24).
Guidelines
Class I Recommendations for ICD implantation (11, 24):
•ERP (Early repolarization pattern): Defined as J-point elevation >1 mm in ≥2 contiguous inferior and/or lateral leads.
•ERS (Early repolarization syndrome): Confirmed in patients with: a resuscitated VF or polymorphic VT and ERP is present on ECG. This combination (a documented arrhythmic event + ERP) confirms the diagnosis of ERS.
Brugada syndrome and SCD risk factors
Brugada syndrome (BrS) is an inherited cardiac channelopathy associated with life-threatening ventricular arrhythmias, including polymorphic VT and VF, leading to an increased risk of SCD (2, 27, 28). Diagnosis is typically based on ECG findings, particularly coved-type ST-segment elevation in the right precordial leads (V1–V3), sometimes accompanied by right bundle branch block (RBBB). Arrhythmic events often occur spontaneously, triggered by VF episodes (29, 30).
Genetics and Pathophysiology
BrS is frequently linked to mutations in the SCN5A gene, which is also associated with LQT3. The pathophysiological mechanism involves the loss of the action potential dome in the right ventricular epicardium (but not the endocardium), leading to transmural dispersion of repolarization and phase 2 reentry, promoting VF (30, 31).
Because the ECG pattern can be concealed, sodium channel blockers such as ajmaline, flecainide, and procainamide are used during pharmacologic testing to unmask the Brugada phenotype (30-32). ICD implantation remains the only proven preventive therapy for SCD in high-risk patients (29, 30, 33).
ECG patterns and diagnosis (see Fig. 3 and Table 1)
|
Table 1. Summary table of risk factors in Brugada syndrome (30) |
||
|---|---|---|
|
Risk factor |
Sudden death risk |
Strength of evidence |
|
Previous cardiac arrest |
Very high |
Strong |
|
Spontaneous type 1 ECG |
High |
Strong |
|
Syncope |
Moderate to high |
Moderate |
|
Inducibility on EPS |
Moderate |
Moderate |
|
Family history of SCD |
Variable |
Weak to moderate |
|
Fever |
Trigger factor |
Moderate |
|
Male sex |
Higher incidence |
Strong |
|
ECG – electrocardiogram, EPS – electrophysiological study, SCD- sudden cardiac death |
||
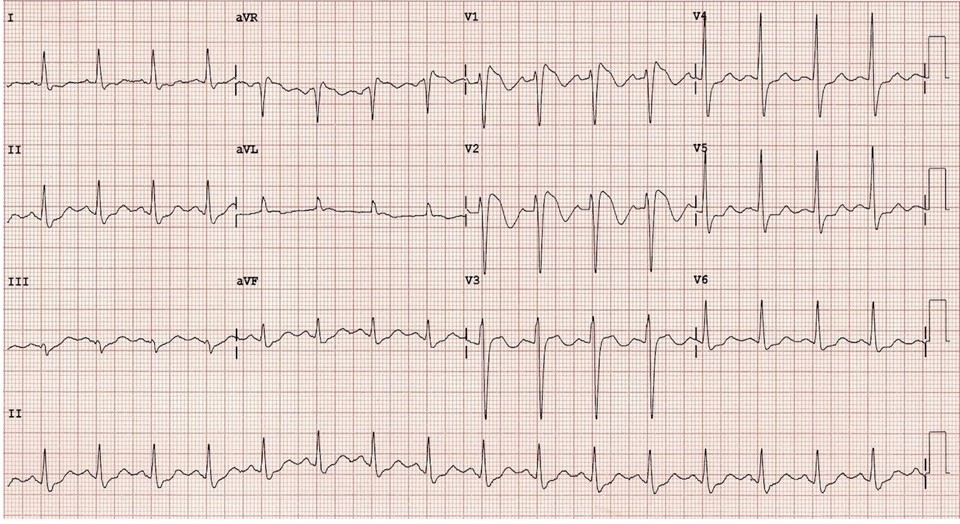
Figure 3. ECG showing BrS with coexisting early repolarization—prominent J-point elevation, coved ST-segment elevation, and inverted T-waves in V1–V2 (2, 27)
BrS – Brugada syndrome, ECG- electrocardiogram
(ECG – authors` collection)
Note: These ECG types are phenotypic expressions. Patients may shift between types depending on factors such as fever, medications, or autonomic changes (29- 33).
Major SCD risk predictors in Brugada Syndrome (30–33)
1. History of sudden cardiac arrest or documented VF – strongest predictor of future events.
2. Spontaneous Type I ECG pattern – carries a higher risk than drug-induced Type I.
3. Syncope – especially if of arrhythmic origin (e.g., transient VT or VF).
4. Inducible VT/VF during electrophysiological study (EPS) – controversial but may suggest increased risk.
5. Family history of SCD before age 45 – raises clinical suspicion; predictive value remains variable.
6. Male sex – BrS is more prevalent and arrhythmogenic in males, possibly due to hormonal or ion channel differences.
7. Fever – can unmask the Brugada ECG pattern and trigger arrhythmias, particularly in children and young adults (28, 29).
Summary
BrS is a high-risk electrical disorder with well-defined ECG criteria and a known genetic basis. Key markers such as a spontaneous type I pattern, arrhythmic syncope, or prior VF should prompt strong consideration of ICD implantation for the prevention of SCD.
Guidelines and risk stratification
Predictors of sudden death in BrS include:
•Inducible VF during programmed stimulation
•History of resuscitated sudden cardiac arrest
•Family history of SCD
•Spontaneous type I ECG, particularly when combined with early repolarization
•Fragmented QRS
•Arrhythmic syncope (29, 31, 32)
Recommendations:
•ICD implantation is indicated for BrS patients with a history of cardiac arrest or sustained VT (Class I) (29, 32).
•ICD should be considered in patients with a spontaneous Type I ECG pattern and arrhythmic syncope (Class IIa) (29, 30, 32)
•A provoked Type I ECG alone (drug-induced) does not establish a diagnosis (29).
•Risk stratification in asymptomatic patients with spontaneous Type I ECG remains controversial (29, 31, 32)
•Routine catheter ablation is not recommended in asymptomatic BrS patients (29, 32)
Long QT syndrome (LQTS)
LQTS is a genetic or acquired condition characterized by QT interval prolongation on ECG, which increases the risk of Torsades de Pointes and SCD (5, 6, 12, 34). A normal corrected QT (QTc) interval is approximately 0.44 seconds. The upper limits are considered 0.46 s for men and 0.47 s for women, with a physiological variation of ±15% (35). Prolonged QT is strongly associated with ventricular arrhythmias. Additional ECG findings, such as T-wave notching, may further support the diagnosis (5, 6).
Forms of LQTS
1. Inherited (Familial):
- Jervell and Lange-Nielsen Syndrome: Autosomal recessive; associated with congenital sensorineural hearing loss (7)
-Romano-Ward syndrome: Autosomal dominant; typically presents with normal hearing (7).
2. Acquired (Non-familial)
-Usually secondary to medications or electrolyte disturbances; no family history (5, 6).
Major LQTS Subtypes (5,7)
•LQTS1 (KCNQ1): ~50% of cases
•LQTS2 (KCNH2): 35–40% of cases
•LQTS3 (SCN5A): <10%; involves impaired sodium inactivation
•Rare types:
-LQTS7 (Andersen–Tawil syndrome)
-LQTS8 (Timothy syndrome)
These subtypes often involve multisystem features.
Common triggers by subtype: LQTS1 - physical exertion, especially swimming, LQTS2 - sudden auditory stimuli or emotional stress, LQTS3 - rest or sleep (5, 6).
ECG patterns by subtype:
•LQTS1: Broad, symmetrical T-waves
•LQTS2: Low-amplitude, notched T-waves
•LQTS3: Prolonged ST segment with relatively normal T-waves (5, 6)
Risk predictors for SCD in LQTS (5, 6, 12-15, 34, 35):
•QTc >500 ms
•History of unexplained syncope
•Trigger exposure consistent with gene subtype (see above)
•Sex differences:
-Higher risk in males during childhood
-Higher risk in females after puberty (12, 14, 15)
•Positive family history of SCD
•Drug-induced QT prolongation or electrolyte imbalance
•High-risk ECG features (e.g., biphasic or notched T-waves)
•Prior history of Torsades de Pointes or cardiac arrest
Management and prevention
Risk assessment
Early identification of high-risk features allows for timely and individualized management (5, 6, 15).
Device therapy
ICD implantation is indicated in:
-Survivors of cardiac arrest
-Patients with recurrent syncope despite medical therapy (5, 6)
Medications
-Beta-blockers (e.g., nadolol, propranolol) are first-line treatment
-Mexiletine may be beneficial in LQTS3
-Avoid QT-prolonging drugs
-Correct electrolyte disturbances (potassium, magnesium, calcium)
Lifestyle modifications
-Avoid known triggers (e.g., swimming in LQTS1, loud noises in LQTS2)
-Restrict participation in competitive sports as appropriate
Family screening and genetic counseling
-All first-degree relatives should undergo clinical and genetic evaluation if a familial mutation is identified (7)
Guideline-Based Recommendations
•Diagnostic criteria include QTc ≥480 ms on serial ECGs (with or without symptoms)
•Use of LQTS clinical scores and genetic testing aids diagnosis
•Asymptomatic carriers should be assessed using tools such as the 1-2-3 Risk Score to guide decision-making (5, 6)
Essential preventive measures
•Avoid medications known to prolong QT interval
•Maintain normal serum electrolytes
•Tailor preventive strategies to the specific genetic subtype
•In high-risk patients, combined therapy with ICD and beta-blockers is often indicated (5, 6)
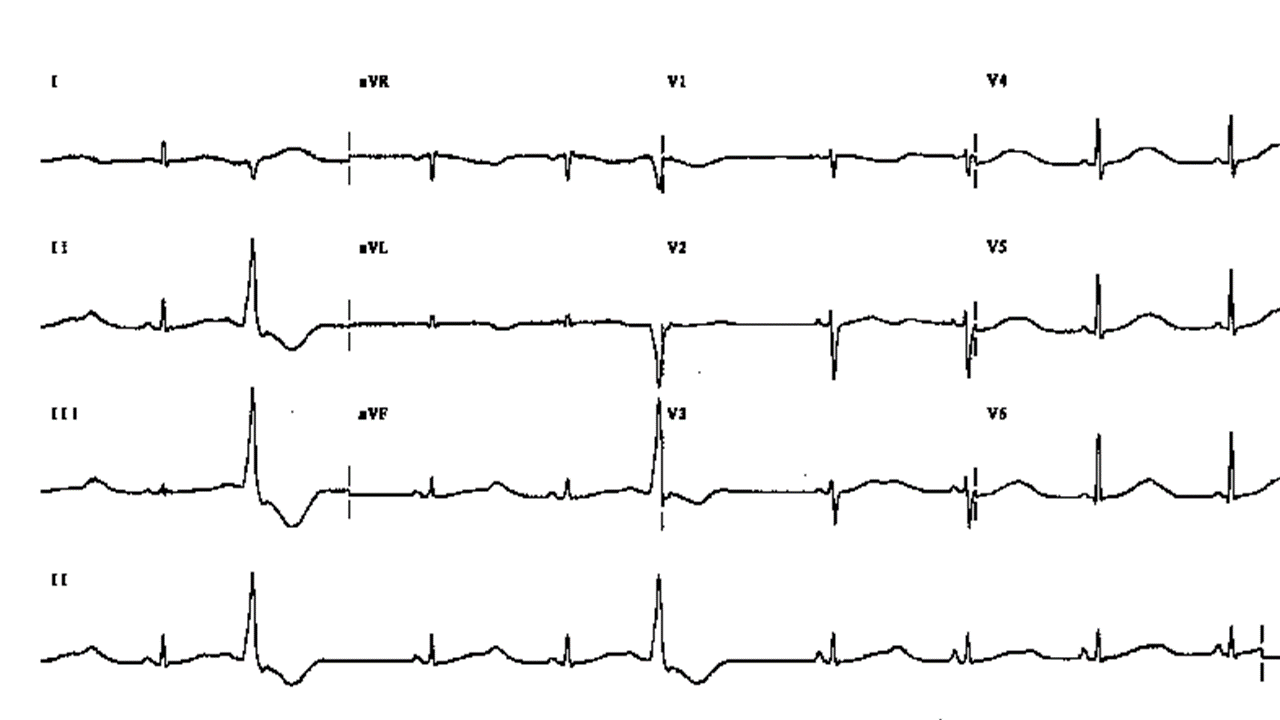
Figure 4. Long QT Syndrome, note bifid T in V3, two ventricular premature beats
Wolff–Parkinson–White Syndrome (WPW)
Wolff–Parkinson–White (WPW) syndrome is a pre-excitation disorder (Fig. 5), typically congenital, though familial cases are rare. Most cases occur sporadically (11).
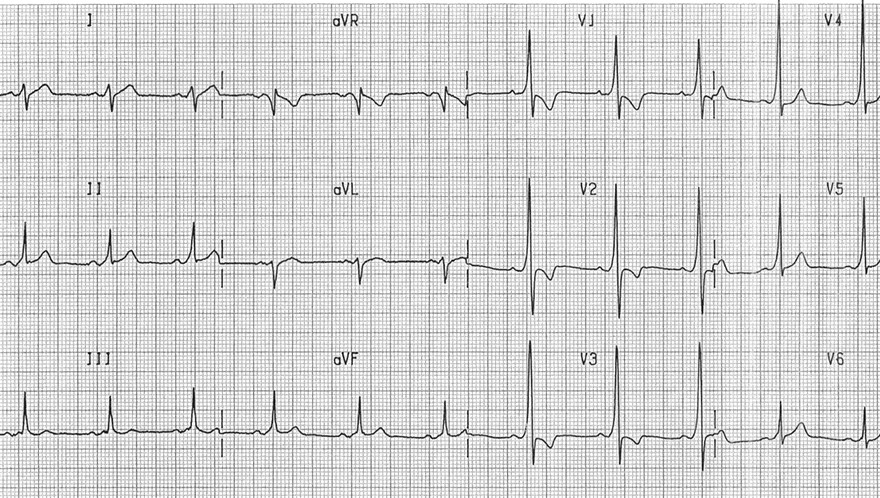
Figure 5. ECG of WPW-syndrome. Note short PR, Delta wave in LII, V2, V3, V4, V5
WPW – Wolf –Parkinson-White syndrome
Mechanism of SCD in WPW:
AF that conducts rapidly through an accessory pathway can result in ventricular rates >300 bpm. The presence of very short RR intervals and high beat-to-beat variability can lead to degeneration into VF and sudden cardiac death (SCD) (5, 6, 11, 34).
Predictors of SCD in WPW (1, 3, 5, 6, 11, 34-37):
•Symptomatic tachyarrhythmias ( atrial fibrillation, AF, supraventricular tachycardia, SVT)
•Shortest pre-excited RR interval <250 ms during AF
•Presence of multiple accessory pathways
•Inducibility of AF or atrioventricular reentry tachycardia (AVRT) during EPS
•Male sex, particularly in individuals under 35 years
•Family history of SCD
•Unexplained syncope
•History of resuscitated cardiac arrest (strongest predictor)
•Inducible VF during EPS
•Short antegrade effective refractory period (<250 ms)
•AF triggered by AVRT during EPS
Demographic Risk Factors
•Male sex
•Young age (<35 years)
•Prior history of AF or AVRT
Risk-Based Management Strategies
•High-risk patients (e.g., with syncope, symptomatic arrhythmias, or high-risk findings on EPS):
Catheter ablation is recommended (1, 5, 11).
•Asymptomatic patients:
Generally considered low risk; however, EPS may be considered in select cases:
-High-risk occupations (e.g., pilots, firefighters)
-Competitive athletes
-If the shortest pre-excited RR interval <250 ms during AF is suspected
Treatment of AVRT in WPW (1)
1.Hemodynamically unstable patients:
Immediate synchronized cardioversion (Class I recommendation)
2.Hemodynamically stable patients:
-Initial approach: Vagal maneuvers (e.g., supine leg raise)
-If unsuccessful: Administer intravenous adenosine (6–18 mg/kg) for orthodromic AVRT (Class I recommendation)
Catecholaminergic polymorphic ventricular tachycardia (CPVT)
CPVT is a rare inherited cardiac channelopathy characterized by stress-induced ventricular arrhythmias, typically occurring in structurally normal hearts with normal resting ECGs. It often presents in childhood or adolescence, with symptoms such as syncope or sudden cardiac arrest during exercise or emotional stress (5, 6, 12-14, 34, 36).
Etiology and genetics
•Autosomal dominant: Most commonly due to mutations in the RYR2 gene
•Autosomal recessive: CASQ2 mutations
•Other associated genes: CALM1, TRDN (7)
Pathophysiology
Adrenergic stimulation (e.g., exercise, emotional stress) leads to abnormal calcium release from the sarcoplasmic reticulum via dysfunctional ryanodine receptors. This triggers delayed afterdepolarizations (DADs) and initiates bidirectional or polymorphic ventricular tachycardia (5-7).
Clinical presentation
•Typical onset: Childhood or adolescence
•Symptoms:
-Syncope (with exertion or emotional stress)
-Palpitations
-Sudden cardiac arrest (in some cases)
•Family history: Sudden cardiac death or unexplained early death (5, 6, 12, 15, 38)
ECG and provocative testing (Fig. 6)
•Resting ECG: Typically normal
•Exercise testing or isoproterenol challenge may reveal:
-Bidirectional VT (alternating QRS axis)
-Polymorphic VT
-Exercise-induced ventricular ectopy (5, 6)
Diagnostic red flags
•Syncope or ventricular arrhythmias triggered by exertion or emotion, despite a normal ECG
•Bidirectional or polymorphic VT during stress testing
•Strong family history of arrhythmias or SCD
•Known RYR2 or CASQ2 mutation carriers (7)
Management Strategies
A. Lifestyle Modifications
•Avoid:
-Competitive or intense physical activity
-Emotional stress and high-stimulation environments
-Stimulants (e.g., caffeine, decongestants) (5, 6, 36)
B. Medical Therapy
•First-line: Non-selective beta-blockers (nadolol or propranolol) (5, 6, 11)
•Adjunct therapy: Flecainide to suppress arrhythmias (5, 6)
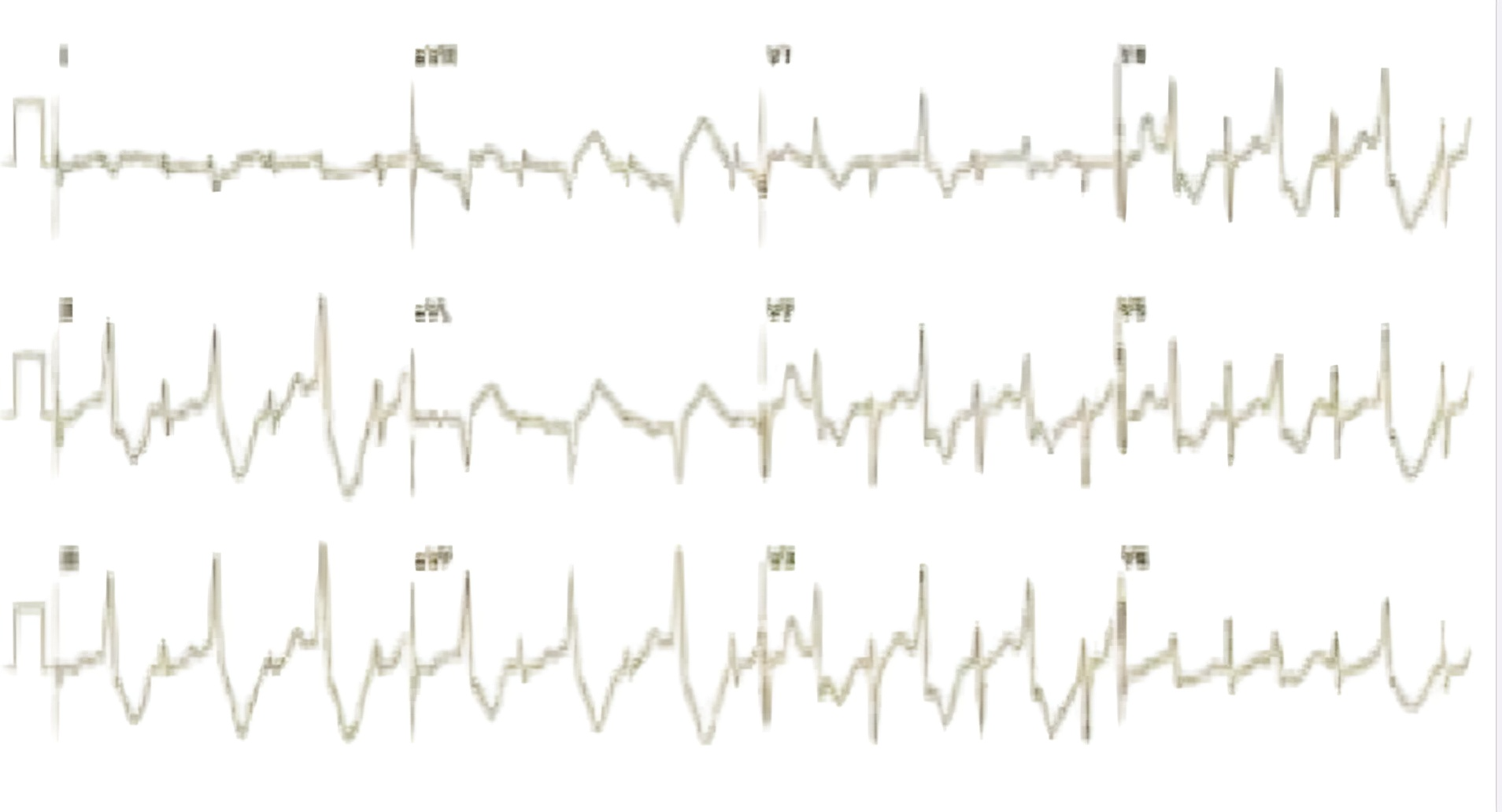
Figure 6. A 12-lead ECG showing bidirectional ventricular tachycardia, characterized by alternating QRS axis and a right bundle branch block (RBBB) morphology, suggesting a left ventricular origin
ECG – electrocardiogram
C. Device Therapy – ICD
•Indicated in:
-Survivors of cardiac arrest
-Patients with breakthrough arrhythmias despite optimal medical therapy
•Caution: High risk of electrical storm and inappropriate shocks; ICDs must be used with careful programming and adjunctive therapy (5, 6, 11)
D. Left cardiac sympathetic denervation (LCSD)
•Consider in cases of:
-Refractory arrhythmias despite beta-blockers and/or ICD
-Complications related to ICD shocks
•Mechanism: Reduces adrenergic input to the heart, lowering arrhythmic risk (5, 6)
Prognosis and guidelines
•Untreated CPVT carries a high risk of SCD
•Appropriate treatment significantly improves long-term outcomes (11-14, 34, 36)
Guideline-based recommendations (ESC & AHA/ACC/HRS)
•Class I diagnosis is supported in patients with:
-Structurally normal hearts and normal resting ECG
-Bidirectional or polymorphic VT induced by exercise or emotional stress (5, 6, 11)
•Genetic testing is recommended for RYR2 or CASQ2 mutations (7)
•Avoidance of competitive sports and stress is advised
•First-line treatment: Non-selective beta-blockers
•ICD implantation is indicated for cardiac arrest survivors, in combination with medications ± flecainide (11, 38, 39)
Conclusion
Inherited arrhythmogenic syndromes such as CPVT are significant contributors to SCD, especially in young individuals and athletes. While often underdiagnosed, these conditions are not rare. Clinical vigilance is essential, particularly in patients with unexplained syncope or exertional arrhythmias. Early recognition enables effective risk stratification and implementation of life-saving interventions.
Peer-review: External and internal
Conflict of interest: None to declare
Authorship: S.M. R. concept and design of the review, writing, M.E.N., M.I.S., M.A.D., A.A.E., A.A.A., M.A.Z., and H.S.A. participated in the collection of literature data, its analysis, and approved the final version to publication, thus equally contributed to preparation of manuscript and fulfilled authorship criteria
Acknowledgement and funding: None to declare
Statement on A.I.-assisted technologies use: The authors did not use AI-assisted technologies in preparation of this manuscript
Data and material availability: Does not apply
References
| 1.Brugada J, Katritsis DG, Arbelo E, Arribas F, Bax JJ, Blomström-et al.; ESC Scientific Document Group. 2020 ESC Guidelines for the management of patients with supraventricular tachycardia. Eur Heart J 2020; 41: 655-720. doi:10.1093/eurheartj/ehz467 https://doi.org/10.1093/eurheartj/ehz467 PMid:31504425 |
||||
| 2.Haïssaguerre M, Nademanee K, Hocini M, Cheniti G, Duchateau J, Frontera A, et al. Depolarization versus repolarization abnormality underlying inferolateral J-wave syndromes: new concepts in sudden cardiac death with apparently normal hearts. Heart Rhythm 2019; 16: 781-90. doi:10.1016/j.hrthm.2018.10.040 https://doi.org/10.1016/j.hrthm.2018.10.040 PMid:30391571 PMCid:PMC6486498 |
||||
| 3.Wilde AAM, Semsarian C, Marquez MF, Shamloo AS, Ackerman MJ, Ashley E, et al. EHRA/HRS/APHRS/LAHRS expert consensus statement on the state of genetic testing for cardiac diseases. Heart Rhythm 2022; 19: e1-e60. doi:10.1016/j.hrthm.2021.09.003 https://doi.org/10.1016/j.hrthm.2021.09.003 PMid:34508877 |
||||
| 4.Stiles MK, Wilde AAM, Abrams DJ, Ackerman MJ, Albert CM, Behr ER, et al. 2020 APHRS/HRS expert consensus statement on the investigation of decedents with sudden unexplained death and patients with sudden cardiac arrest, and of their families. Heart Rhythm 2021; 18: e1-e50. doi:10.1016/j.hrthm.2020.10.010 https://doi.org/10.1016/j.hrthm.2020.10.010 PMid:33091602 PMCid:PMC8194370 |
||||
| 5.Priori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J 2015; 36: 2793-867. doi:10.1093/eurheartj/ehv316 https://doi.org/10.1093/eurheartj/ehv316 PMid:26320108 |
||||
| 6. Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, et al. 2017 AHA/ACC/HRS Guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Circulation 2018; 138: e272-e391. doi:10.1161/CIR.0000000000000549 https://doi.org/10.1161/CIR.0000000000000549 |
||||
| 7. Cerrone M, Priori SG. Genetics of sudden death: focus on inherited channelopathies. Eur Heart J 2011; 32: 2109-118. doi:10.1093/eurheartj/ehr041 https://doi.org/10.1093/eurheartj/ehr041 PMid:21362704 |
||||
| 8. Ali A, Butt N, Sheikh AS. Early repolarization syndrome: a cause of sudden cardiac death. World J Cardiol 2015; 7: 466-475. doi:10.4330/wjc.v7.i8.466 https://doi.org/10.4330/wjc.v7.i8.466 PMid:26322186 PMCid:PMC4549780 |
||||
| 9.Moore BM, Roston TM, Laksman Z, Krahn AD. Updates on inherited arrhythmia syndromes (Brugada syndrome, long QT syndrome, CPVT, ARVC). Prog Cardiovasc Di. 2025; S0033-0620(25)00079-9. doi:10.1016/j.pcad.2025.06.010 https://doi.org/10.1016/j.pcad.2025.06.010 PMid:40614914 |
||||
| 10.Moisa SM, Spoiala EL, Cinteza E, Vatasescu R, Butnariu LI, et al. Arrhythmogenic right ventricular cardiomyopathy in children: a systematic review. Diagnostics (Basel) 2024; 14: 175. doi:10.3390/diagnostics14020175 https://doi.org/10.3390/diagnostics14020175 PMid:38248052 PMCid:PMC10814764 |
||||
| 11.Zeppenfeld K, Tfelt-Hansen J, de Riva M, Winkel BG, Behr ER, Blom NA, et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J 2022; 43: 3997-4126. doi:10.1093/eurheartj/ehac262 https://doi.org/10.1093/eurheartj/ehac262 PMid:36017572 |
||||
| 12. Hayashi M, Shimizu W, Albert CM. The spectrum of epidemiology underlying sudden cardiac death. Circ Res 2015; 116: 1887-906. doi:10.1161/CIRCRESAHA.116.304521 https://doi.org/10.1161/CIRCRESAHA.116.304521 PMid:26044246 PMCid:PMC4929621 |
||||
| 13. Chugh SS, Reinier K, Teodorescu C, Evanado A, Kehr E, Al Samara M, et al. Epidemiology of sudden cardiac death: clinical and research implications. Prog Cardiovasc Dis 2008; 51: 213-28. doi:10.1016/j.pcad.2008.06.003 https://doi.org/10.1016/j.pcad.2008.06.003 PMid:19026856 PMCid:PMC2621010 |
||||
| 14. Reinier K, Stecker EC, Uy-Evanado A, Chugh HS, Binz A, et al. Sudden cardiac death as first manifestation of heart disease in women: the Oregon Sudden Unexpected Death Study, 2004-2016. Circulation 2020; 141: 606-8. doi:10.1161/CIRCULATIONAHA.119.044169 https://doi.org/10.1161/CIRCULATIONAHA.119.044169 PMid:32065764 PMCid:PMC7122833 |
||||
| 15. Wellens HJJ, Schwartz PJ, Lindemans FW, Buxton AE, Goldberger JJ, Hohnloser SH, et al. Risk stratification for sudden cardiac death: current approaches and emerging challenges. Nat Rev Cardiol 2014; 11: 541-9. doi:10.1038/nrcardio.2014.78 https://doi.org/10.1038/nrcardio.2014.78 PMid:24889520 |
||||
| 16. Ji HY, Hu N, Liu R, Zhou HR, Gao WL, Quan XQ. Worldwide prevalence of early repolarization pattern in the general population and physically active individuals. Medicine (Baltimore) 2021; 100: e25978. doi:10.1097/MD.0000000000025978 https://doi.org/10.1097/MD.0000000000025978 PMid:34087840 PMCid:PMC8183793 |
||||
| 17.Yakkali S, Teresa Selvin S, Thomas S, Bikeyeva V, Abdullah A, Radivojevic A, et al. why is there an increased risk for sudden cardiac death in patients with early repolarization syndrome? Cureus 2022; 14: e26820. doi: 10.7759/cureus.26820 https://doi.org/10.7759/cureus.26820 |
||||
| 18.Higgins JP. Normal resting electrocardiographic variants in young athletes. Phys Sportsmed 2008; 36: 69-75. doi:10.3810/psm.2008.12.14 https://doi.org/10.3810/psm.2008.12.14 PMid:20048474 |
||||
| 19.Wellens HJJ. Early repolarization revisited. N Engl J Med 2008; 358: 2063-65. doi:10.1056/NEJMe0802124 https://doi.org/10.1056/NEJMe0801060 PMid:18463384 |
||||
| 20.Mehta MC, Jain AC. Early repolarization on a scalar electrocardiogram. Am J Med Sci 1995; 309: 305-11. doi:10.1097/00000441-199506000-00001 https://doi.org/10.1097/00000441-199506000-00001 PMid:7771499 |
||||
| 21.Gussak I, Antzelevitch C. Early repolarization syndrome: clinical characteristics and possible cellular and ionic mechanisms. J Electrocardiol 2000; 33: 299-309. doi:10.1054/jelc.2000.20394 https://doi.org/10.1054/jelc.2000.18106 PMid:11099355 |
||||
| 22.Haïssaguerre M, Derval N, Sacher F, Jesel L, Deisenhofer I, de Roy L, et al. Sudden cardiac arrest associated with early repolarization. N Engl J Med 2008; 358: 2016-23. doi:10.1056/NEJMoa071968 https://doi.org/10.1056/NEJMoa071968 PMid:18463377 |
||||
| 23.Tikkanen JT, Anttonen O, Junttila MJ, Aro AL, Kerola T, Rissanen HA, et al. Long-term outcome associated with early repolarization on electrocardiography. N Engl J Med 2009; 361: 2529-537. doi:10.1056/NEJMoa0907589 https://doi.org/10.1056/NEJMoa0907589 PMid:19917913 |
||||
| 24.Antzelevitch C. J wave syndromes: molecular and cellular mechanisms. J Electrocardiol 2013; 46: 510-8. doi: 10.1016/j.jelectrocard.2013.08.006 | ||||
| 25.Samad A, Thakar HP, Hassan M, Kaur J, Hussein AT. Navigating diagnostic difficulties: benign early repolarization/subtle ST elevation in a young patient presenting as myocardial infarction. Cureus 2025; 17: e89632. doi:10.7759/cureus.89632 https://doi.org/10.7759/cureus.89632 |
||||
| 26. Antzelevitch C., Yan G.X. J-wave syndromes: Brugada and early repolarization syndromes. Heart Rhythm 2015; 12: 1852-66. doi: 10.1016/j.hrthm.2015.04.014 https://doi.org/10.1016/j.hrthm.2015.04.014 PMid:25869754 PMCid:PMC4737709 |
||||
| 27.Sarkozy A, Chierchia GB, Paparella G, Brugada P. Inferior and lateral electrocardiographic repolarization abnormalities in Brugada syndrome. Circ Arrhythm Electrophysiol 2009; 2: 154-7. doi:10.1161/CIRCEP.108.816660 https://doi.org/10.1161/CIRCEP.108.795153 PMid:19808460 |
||||
| 28.Roterberg G, El‐Battrawy I, Veith M, Liebe V, Ansari U, Lang S, et al. Arrhythmic events in Brugada syndrome patients induced by fever. Ann Noninvasive Electrocardiol. 2019; 25: e12723. doi: 10.1111/anec.12723 | ||||
| 29.Antzelevitch C, Brugada P, Borggrefe M, Brugada J, Brugada R, Corrado D, et al. Brugada syndrome: report of the second consensus conference: endorsed by the Heart Rhythm Society and the European Heart Rhythm Association. Circulation 2005; 111: 659-70. doi:10.1161/01.CIR.0000152479.54298.51 https://doi.org/10.1161/01.CIR.0000152479.54298.51 PMid:15655131 |
||||
| 30.Batchvarov VN. The Brugada syndrome - diagnosis, clinical implications and risk stratification. Eur Cardiol 2014; 9: 82-7. doi:10.15420/ecr.2014.9.2.82 https://doi.org/10.15420/ecr.2014.9.2.82 PMid:30310491 PMCid:PMC6159405 |
||||
| 31.Naseef A, Behr ER, Batchvarov VN. Electrocardiographic methods for diagnosis and risk stratification in the Brugada syndrome. J Saudi Heart Assoc 2015; 27: 96-108. doi:10.1016/j.jsha.2014.07.003 https://doi.org/10.1016/j.jsha.2014.07.003 PMid:25544823 PMCid:PMC4274309 |
||||
| 32.Probst V, Veltmann C, Eckardt L, Meregalli PG, Gaita F, Tan HL, et al. Risk stratification in Brugada syndrome: results of the FINGER Brugada Syndrome Registry. Circulation 2010; 121: 635-43. doi:10.1161/CIRCULATIONAHA.109.878652 https://doi.org/10.1161/CIRCULATIONAHA.109.887026 PMid:20100972 |
||||
| 33. Huikuri HV, Castellanos A, Myerburg RJ. Sudden death due to cardiac arrhythmias. N Engl J Med 2001; 345: 1473-82. doi:10.1056/NEJMra000650 https://doi.org/10.1056/NEJMra000650 PMid:11794197 |
||||
| 34.Lazzoli JK, Soares PP, da Nobrega AC, de Araujo CG. Electrocardiographic criteria for vagotonia: validation with pharmacological parasympathetic blockade in healthy subjects. Int J Cardiol 2003; 87:231-6. doi:10.1016/S0167-5273(02)00418-1 https://doi.org/10.1016/S0167-5273(02)00330-3 PMid:12559544 |
||||
| 35. Maron BJ. Sudden death in young athletes. N Engl J Med 2003; 349: 1064-75. doi:10.1056/NEJMra022783 https://doi.org/10.1056/NEJMra022783 PMid:12968091 |
||||
| 36.Sarquella-Brugada G, Campuzano O. Inherited arrhythmogenic syndromes. Cardiogenetics 2023; 13: 173-4. doi:10.3390/cardiogenetics13040017 https://doi.org/10.3390/cardiogenetics13040016 |
||||
| 37. Rafla S, Kamal A, Younes A, Abdelsalam Y. The significance of early repolarization and incomplete right bundle block in athletes. Int J Sports Exerc Med 2020; 6: 168. doi:10.23937/2469-5718/1510168 https://doi.org/10.23937/2469-5718/1510168 |
||||
| 38.Janzen ML, Davies B, Laksman ZWM, Roberts JD, Sanatani S, Steinberg C, et al. Management of inherited arrhythmia syndromes: a HiRO consensus handbook on process of care. CJC Open 2023; 5: 268-84. doi:10.1016/j.cjco.2023.02.008 https://doi.org/10.1016/j.cjco.2023.02.008 PMid:37377520 PMCid:PMC10290944 |
||||
| 39.Cronin EM, Bogun FM, Maury P, Peichl P, Chen M, Namboodiri N, et al. 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias. Europace. 2019; 21:1143-244. doi:10.1093/europace/euz132 https://doi.org/10.1093/europace/euz132 PMid:31075787 PMCid:PMC7967791 |
||||
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

