The Chagas storm - an uncommon presentation of the disease: A case report and literature review
CASE REPORT
The Chagas storm - an uncommon presentation of the disease: A case report and literature review
Article Summary
- DOI: 10.24969/hvt.2025.608
- CARDIOVASCULAR DISEASES
- Published: 24/11/2025
- Received: 10/09/2025
- Revised: 27/09/2025
- Accepted: 27/09/2025
- Views: 661
- Downloads: 435
- Keywords: Chagas disease, chronic Chagas cardiomyopathy, electric storm, aneurysmectomy, case report
Address for Correspondence: Edgar Fránquez Flores, Cardiology Department, Mexican Institute of Social Security; UMAE of Specialties CMN General Manuel Avila Camacho, Puebla, México
Email: edgarfranquez20@outlook.com Mobile: +52 3111020465
ORCID: Edgar Jesús Fránquez-Flores: 0009-0004-1265-6426; Eduardo Sánchez-Cortes: 0009-0005-7264-3237
Edgar Jesús Fránquez-Flores1, Edgar Michael Lizárraga-Maldonado1, Rosa Elena Gutiérrez-Castañeda, Juan Guzmán-Olea2, Jorge Guillermo Arenas-Fonseca1, Eduardo Sánchez-Cortes1, Gabriel Guzmán Olea1
1Cardiology Department, Mexican Institute of Social Security; UMAE of Specialties CMN General Manuel Avila Camacho, Puebla, México.
2Hospital Angeles, Puebla, Mexico
Abstract
Objective: We present as case of rare manifestation of electrical storms due to Chagas cardiomyopathy, the complexity of its management, and how to resolve them.
Case presentation: Female patient with no cardiovascular history, was admitted to the hospital with palpitations, dyspnea, diaphoresis and atypical oppressive chest pain. In the emergency department, she was diagnosed with ventricular tachycardia (VT) with hemodynamic stability, doctors decided to start pharmacological cardioversion with intravenpusly without clinical improvement or achieving cardioversion, and then after detecting an evolution in hemodynamic instability, electrical cardioversion was performed at 200 J with return to sinus rhythm. However, she experienced multiple recurrences of VT, so due to suspicion of ischemic etiology, she underwent diagnostic coronary angiography where epicardial arteries were reported without significant lesions and imaging suggestive of inferior basal aneurysm. Transthoracic echocardiogram revealed left ventricle dilated, left ventricular ejection fraction of 49% and possible Chagas-related cardiomyopathy due to an aneurysm was suspected and serology for Chagas was requested with a “reactive” result, confirming the diagnosis. The patient is currently under treatment with class III antiarrhythmic and beta-blocker with adequate adherence and follow-up without any electrical instability so far.
Conclusion: In hemodynamically unstable patients with Chagas disease and electrical storm, intravenous antiarrhythmics are the initial choice. Ablation is effective in controlling recurrent VT (electric storm) as a definitive therapy. Combined endocardial/epicardial catheter ablation strategies reduce VT/ventricular fibrillation recurrences but do not cure the disease.
Key words: Chagas disease, chronic Chagas cardiomyopathy, electric storm, aneurysmectomy, case report
Introduction
The Chagas disease is infection caused by the parasite Trypanosoma cruzi. It is highly prevalent in areas such as Central and South America, where it is responsible for serious heart disease that can be fatal if not treated in time (8).
Chagas cardiomyopathy is defined as the presence of typical electrocardiographic abnormalities associated with a positive serology for Trypanosoma cruzi. According to the natural history of the disease, more than 50% of patients infected with the protozoan may live asymptomatically. However, once the disease manifests itself, electrical conduction disorders and cardiac segmental mobility disorders may be observed. In the most severe cases, it can manifest as acute heart failure, lethal arrhythmias, thromboembolic events, and sudden cardiac death (1, 2).
Graphical abstract

Chronic Chagas disease is subdivided into four clinical presentations, with the indeterminate form being the most common, in which certain predisposing factors such as age, sex, comorbidities, and alcoholism, among others, may promote progression towards cardiac manifestation.
The following is the case of a patient who presented with electric storm, structural cardiac alterations suggestive of chronic Chagas disease, which was confirmed by serology. The significance of this case lies in the rare presentation of electrical storms due to Chagas cardiomyopathy, the complexity of its management, and how to resolve them. Early detection and treatment resulted in good outcomes and quality of life.
Case presentation
A 67-year-old female patient with cardiovascular risk factors (obesity, postmenopausal, age), no cardiovascular history, and a history of fatty liver disease 7 years prior was admitted to the emergency department.
The patient reported severe clinical symptoms with palpitations, dyspnea, diaphoresis, fatigue, weakness, and atypical oppressive chest pain.
Physical examination revealed a grade IV/VI holosystolic murmur in the mitral focus with left scapular extension, increasing in intensity in the left lateral decubitus position.
A 12-lead electrocardiogram was taken (Fig. 1), revealing wide QRS tachycardia, with a heart rate of 196 bpm, with Brugada criteria (absence of RS in precordial leads, R> 30 ms in V1, QRS> 140 ms, axis -60°, complete right bundle branch block (RBBB) morphology with monophasic R, with initial R in aVR) suggestive of ventricular tachycardia (VT) with probable inferior origin.
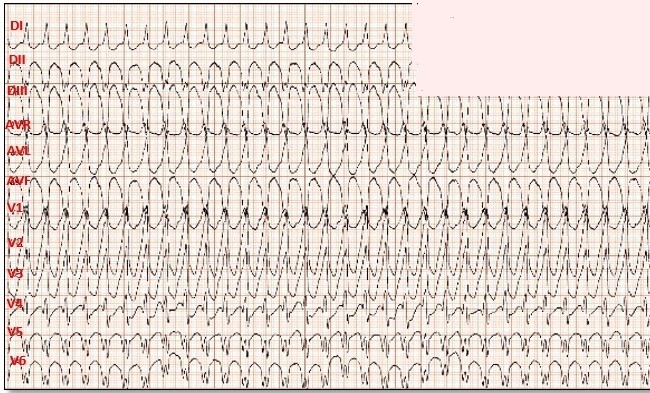
Figure 1. Electrocardiogram. Monomorphic ventricular tachycardia with Brugada criteria (absence of RS in precordial leads, R:30 ms in V1, QRS >140 ms, axis -60°, complete right bundle branch block (RBBB) morphology with monophasic R in AVR)
A chest X-ray revealed grade IV cardiomegaly and a cardiothoracic index of 0.62 (Fig. 2). Laboratory tests revealed elevated cardiac biomarkers, with an initial high-sensitivity troponin I of 16.1 ng/dL and creatine phosphokinase MB fraction of 95; the rest of the laboratory results were within normal ranges.
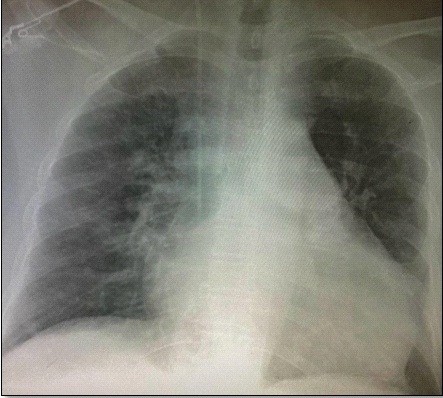
Figure 2. Chest X-RAY. Cardiomegaly with cardiothoracic index of 0.62
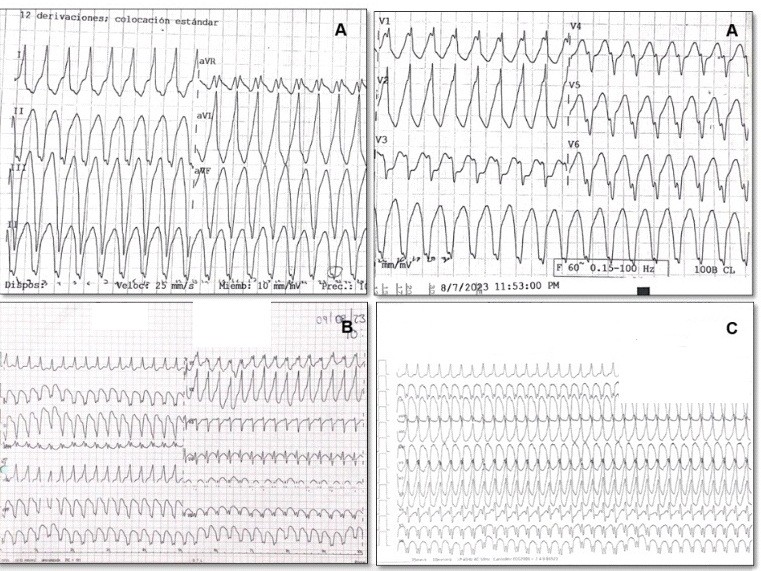
During emergency care, her vital signs remained adequate and stable (hemodynamic stability), so it was decided to initiate pharmacological cardioversion with intravenous amiodarone (300 mg over 20 minutes followed by an infusion of 0.5 mg/min). However, she experienced multiple recurrences of VT (electrical storm) (Fig. 3), and intravenous lidocaine was added to the antiarrhythmic treatment (1 mg/kg bolus followed by an infusion of 1 mg/min). However, there was no clinical improvement, with progression to hemodynamic instability. Electrical cardioversion at 200 J was performed, with a return to sinus rhythm at 54 bpm and the presence of complete RBBB (Fig. 4).
Owing to the suspicion of ischemic etiology (age and risk factors), she underwent diagnostic coronary angiography where epicardial arteries were reported without significant lesions, Markis 4 coronary ectasia, slow pancoronary flow, mitral regurgitation (MR) +++/++++ of Sellers and images suggestive of inferior basal aneurysms, left ventricular ejection fraction (LVEF) of 50%, left ventricular (LV) end-diastolic pressure of 25 mmHg (Fig. 5).
A transthoracic echocardiogram revealed dilated, possibly Chagas-related cardiomyopathy due to an aneurysm involving two segments (basal inferolateral and basal anterolateral), with an aneurysm-to-mouth width ratio of 0.45; the remaining mobility was normal. The LV had an enlarged diameter, eccentric hypertrophy and a 49% LVEF. The mechanism of severe MR was secondary to annular dysfunction due to the aneurysm, in addition mitral annulus dilation and asymmetric tenting of the posterior leaflet, resulted in a wide, eccentric, and complex jet with a Coanda effect. The probability of pulmonary hypertension was low, with a pulmonary artery systolic pressure of 32 mmHg (Fig. 6, 7).
![]()
Figure 3. Multiple episodes of ventricular tachycardia (electrical storm)
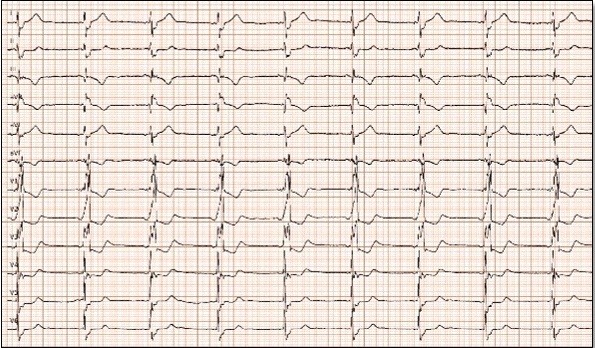
Figure 4. Electrocardiogram after electrical cardioversion. Sinus rhythm at 54 bpm with complete right bundle branch block
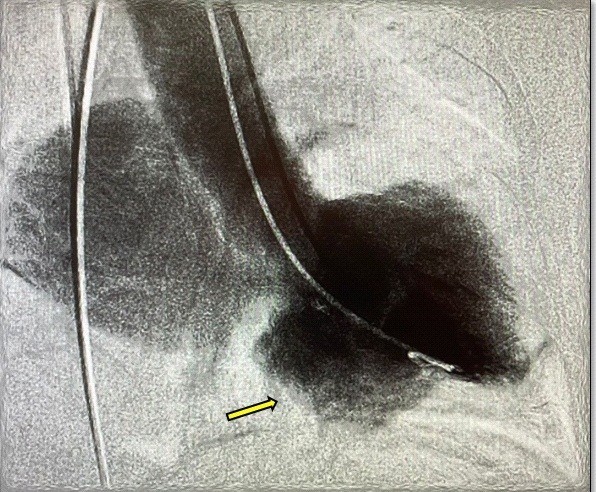
Figure 5. Ventriculogram displays mitral regurgitation of Sellers +++/++++ grade and inferior-basal aneurysm (yellow arrow)
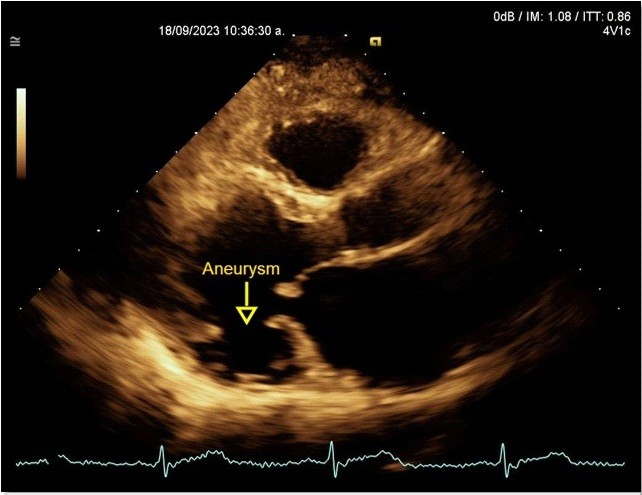
Figure 5. Echocardiogram, parasternal long-axis view: Basal inferior-lateral aneurysm, aneurysm-to-mouth width ratio – 0.45 (Yellow arrow)
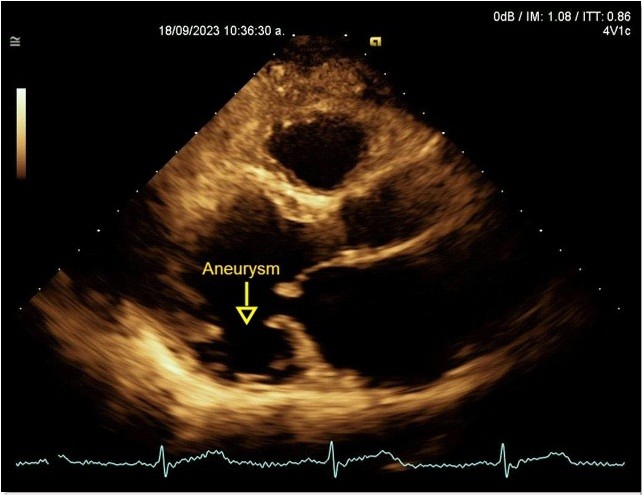
Figure 6. Echocardiogram, parasternal long-axis view: Basal inferior-lateral aneurysm, aneurysm-to-mouth width ratio – 0.45 (Yellow arrow)
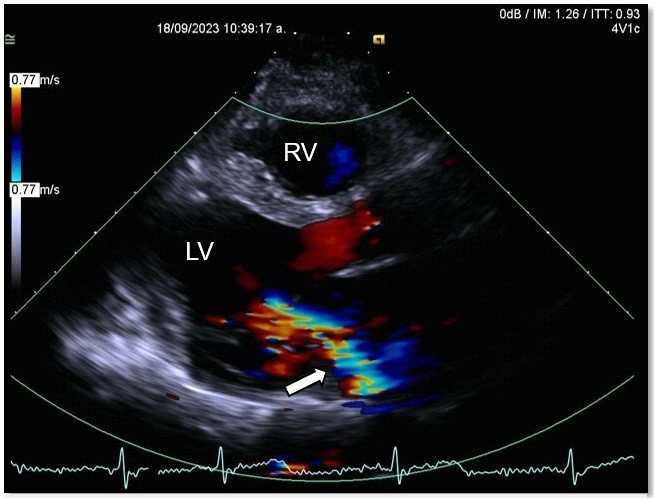
Figure 7. Echocardiogram, parasternal long-axis view: left ventricular ejection fraction=49%, severe mitral regurgitation with large eccentric jet and Coanda effect (white arrow)
The Chagas disease was suspected due to the presence of a large unexplained LV aneurysm, absence of ischemic disease and patient residence in regions where Chagas disease remains prevalent. Serology was requested with a “reactive” result, confirming the diagnosis.
Following analysis of the case by a multidisciplinary team, the patient is being treated with class III antiarrhythmics (amiodarone), beta-blockers (metoprolol) and loop diuretics (furosemide) for mitral regurgitation, with adequate adherence and no electrical instability, and is following a catheter ablation protocol as definitive therapy.
Discussion
Chagas disease remains a global health problem, associated with high morbidity and mortality, with an impact on health services predominantly in Latin America (1).
In Mexico, the prevalence of Chagas disease remains high. In one study, the prevalence was assessed by determining Trypanosoma cruzi serology in the Northern region of the state of Mexico. It was found that, despite the efforts of the health system, there are areas where health programs for prevention, timely identification and timely treatment need to be intensified (2).
Among the differential characteristics that have been described for Chagas cardiomyopathy, is the specific pattern of fibrosis, which is usually located in the posterior and apical regions of the LV. The clinical manifestations are wide-ranging, from asymptomatic subjects to severe forms of the disease such as heart failure, lethal ventricular arrhythmias, embolic events, and sudden cardiac death (3).
There is a high arrhythmogenic substrate in Chagas cardiomyopathy, secondary to the development of myocardial fibrosis and necrotic lesions, manifesting especially with ventricular tachyarrhythmias, followed by atrial tachyarrhythmias and conduction system disorders such as atrioventricular block (4).
Ventricular aneurysms are an additional substrate for arrhythmia and a factor that promotes the formation of mural thrombus, increasing the risk of embolic events. The inferolateral location of the LV is usually the most frequently reported site in the literature, coinciding with the findings in the case presented (5, 6).
About drug therapy, the use of benznidazole and nifurtimox is effective during the acute phase of Chagas disease (7); however, there is insufficient evidence to recommend it in the chronic phases of the disease (7).
In clinical practice, patients presenting with symptomatic arrhythmias are mostly treated with amiodarone despite its adverse effects, due to the lack of randomised clinical trials evaluating efficacy and safety. In our clinical setting, we follow international guidelines that suggest starting with antiarrhythmic drugs such as amiodarone, lidocaine, and procainamide as a first step, and in refractory cases, rescue therapies such as stellate ganglion block, urgent ablation, etc. (4, 7).
However, there are reports of case series in which accumulated experience suggests a benefit in preventing ventricular arrhythmia recurrence, with the greatest benefit seen in patients with a reduced LVEF of less than 41% (8, 9).
For primary prevention, antiarrhythmic drugs have been compared with implantable cardioverter defibrillators (ICDs). Preliminary data from the CHAGASICS study, which compared the use of ICDs with amiodarone alone, indicate that although ICDs do not reduce all-cause mortality, they do have an impact on reducing the incidence of sudden cardiac death and hospitalizations for heart failure, and are therefore considered a treatment alternative in selected cases (10).
For secondary prevention, ICDs have been shown to reduce mortality in patients with chronic Chagas cardiomyopathy who had a history of malignant arrhythmias or aborted sudden death, especially when associated with an LVEF of less than 41% (11).
Innovative emergency therapies aimed at modulating the sympathetic system in patients presenting with refractory electrical storms, such as unilateral or bilateral stellate ganglion block, have been reported in the literature. The safety and efficacy of the therapy was evaluated in the STAR study, demonstrating a significant reduction in arrhythmic burden of 93% (12, 13).
Among ablation therapies, there has been debate about the efficacy of endocardial ablation alone or combined endocardial/epicardial ablation in reducing the percentage of arrhythmias (14, 15). As noted in recent reviews, combined endocardial/epicardial catheter ablation therapy has shown significantly better results in reducing VT recurrences (16).
Our patient is undergoing a protocol for catheter ablation, with a Painesd Score of 8 points, which indicates a low risk of hemodynamic decompensation during VT ablation (16, 4).
Finally, approaches such as ventricular aneurysmectomy, bilateral cervicothoracic sympathectomy, or renal denervation have been proposed as alternatives in patients with persistent electrical storm who do not respond to drugs and in whom ablation is not effective or feasible (17).
Conclusion
Chagas cardiomyopathy remains a very complex condition to treat. In cases presenting with electrical storm and hemodynamic instability, treatment options are limited to antiarrhythmic drugs or interventional rescue therapies. However, due to the multiple arrhythmogenic foci of the disease, a highly experienced team is required to perform the procedures safely. Although ventricular aneurysm has a high incidence in Chagas cardiomyopathy, its association with severe mitral regurgitation increases the risk of heart failure, arrhythmias, or sudden death. Therefore, it is very important to understand the natural history of the disease and the treatment options available to maintain hemodynamic stability, reduce the recurrence of arrhythmias, and improve the prognosis of patients with secondary prevention therapies.
Take home message
- In hemodynamically unstable patients with Chagas electrical storm, intravenous antiarrhythmics are the initial choice.
- Ablation is effective in controlling recurrent ventricular tachycardia (electric storm) as a definitive therapy.
- Combined endocardial/epicardial catheter ablation strategies reduce VT/ventricular fibrillation recurrences but do not cure the disease.
Ethics: The study involving human participants were reviewed and approved by the Ethics Committee of Specialty Hospital Puebla, Mexico (2025). We followed Helsinki 2024 declaration rules for studies on humans.
Informed consent was obtained for patients for all procedures.
Written informed consent was obtained from the patient for publication of this case report.
Peer-review: External and internal
Conflict of interest: None to declare
Authorship: E.F.F. and E.L.M. contributed to the conception and design of the work, and RGC wrote the manuscript. J.G.O., R.G.C. and E.F.F. were participants in the treatment and management of the patient. J.G.A.F. contributed to image acquisition. E.S.C. and G.G.O. was the major contributor to the critical revision of the manuscript. All the authors read and approved the final manuscript and equally contributed to manuscript preparation and fulfilled the authorship criteria
Acknowledgements and funding: None to declare
Statement on A.I.-assisted technologies use: Authors did not use A.I. technology in preparation of manuscript
Data and material availability: No datasets were generated or analyzed during the current study
References
| 1.Eifling M, Razavi M, Massumi A. The evaluation and management of electrical storm. Tex Heart Inst J 2011; 38: 111-21. | ||||
| 2.Padilla-Valdez JM, Antonio-Campos A, Montes-Vergara Y, González-Quiroz JL, Domínguez-López ML, Martínez-Hernández F, et al. Serological determination of Trypanosoma cruzi in Northern region of the State of Mexico. Parasitol Res 2025; 124: 23. doi: 10.1007/s00436-025-08464-6 https://doi.org/10.1007/s00436-025-08464-6 PMid:39964490 PMCid:PMC11835914 |
||||
| 3.Noda T, Kurita T, Nitta T, Chiba Y, Furushima H, Matsumoto N, et al. Significant impact of electrical storm on mortality in patients with structural heart disease and an implantable cardiac defibrillator. Int J Cardiol 2018; 255: 85-91. https://doi.org/10.1016/j.ijcard.2017.11.077 PMid:29425569 |
||||
| doi: 10.1016/j.ijcard.2017.11.077 https://doi.org/10.1016/j.ijcard.2017.11.077 PMid:29425569 |
||||
| 4.Jentzer JC, Noseworthy PA, Kashou AH, May AM, Chrispin J, Kabra R, et al. Multidisciplinary critical care management of electrical storm: JACC state-of-the-art review. J Am Coll Cardiol 2023; 81: 2189-206. Doi: 10.1016/j.jacc.2023.03.424 https://doi.org/10.1016/j.jacc.2023.03.424 PMid:37257955 PMCid:PMC10683004 |
||||
| 5.Ribeiro Cury Pavão ML, Arfelli E, Scorzoni-Filho A, Pavão RB, Pazin-Filho A, Marin-Neto JA, et al. Electrical storm in Chagas cardiomyopathy: Clinical predictors, outcome, and arrhythmic characteristics in a prospective registry. JACC Clin Electrophysiol 2020; 6: 1238-45. Doi: 10.1016/j.jacep.2020.04.028 https://doi.org/10.1016/j.jacep.2020.04.028 PMid:33092749 |
||||
| 6.Johnson MC, Wang L. The imperfect storm: An uncommon presentation of Chagas disease. Am J Emerg Med 2022; 61: 235.e1-e3. doi: 10.1016/j.ajem.2022.08.011 https://doi.org/10.1016/j.ajem.2022.08.011 PMid:35961831 |
||||
| 7.Baldi E, Conte G, Zeppenfeld K, Lenarczyk R, Guerra JM, Farkowski MM, et al. Contemporary management of ventricular electrical storm in Europe: results of a European Heart Rhythm Association Survey. Europace 2023; 25: 1277-83. doi: 10.1093/europace/euac151 https://doi.org/10.1093/europace/euac151 PMid:36196613 PMCid:PMC10105853 |
||||
| 8.Nunes MCP, Beaton A, Acquatella H, Bern C, Bolger AF, Echeverría LE, et al. Chagas cardiomyopathy: An update of current clinical knowledge and management: A scientific statement from the American Heart Association. Circulation 2018; 138: e169-209. Doi: 10.1161/CIR.0000000000000599 https://doi.org/10.1161/CIR.0000000000000599 PMid:30354432 |
||||
| 9.Guarracini F, Bonvicini E, Zanon S, Martin M, Casagranda G, Mochen M, et al. Emergency management of electrical storm: A practical overview. Medicina (Kaunas) 2023; 59. Doi: 10.3390/medicina59020405 https://doi.org/10.3390/medicina59020405 PMid:36837606 PMCid:PMC9963509 |
||||
| 10.Martinelli-Filho M, Marin-Neto JA, Scanavacca MI, de Paola AAV, Medeiros P de TJ, Owen R, et al. Amiodarone or implantable cardioverter-defibrillator in Chagas cardiomyopathy: The CHAGASICS randomized clinical trial: The CHAGASICS randomized clinical trial. JAMA Cardiol 2024; 9: 1073-81. Doi: 10.1001/jamacardio.2024.3169 https://doi.org/10.1001/jamacardio.2024.3169 PMid:39356542 PMCid:PMC11447631 |
||||
| 11.Nademanee K, Taylor R, Bailey WE, Rieders DE, Kosar EM. Treating electrical storm : sympathetic blockade versus advanced cardiac life support-guided therapy: Sympathetic blockade versus Advanced Cardiac Life Support-guided therapy. Circulation 2000; 102: 742-7.doi: 10.1161/01.cir.102.7.742 https://doi.org/10.1161/01.CIR.102.7.742 PMid:10942741 |
||||
| 12.Savastano S, Baldi E, Compagnoni S, Rordorf R, Sanzo A, Gentile FR, et al. Electrical storm treatment by percutaneous stellate ganglion block: the STAR study. Eur Heart J 2024; 45: 823-33. Doi: 10.1093/eurheartj/ehae021 https://doi.org/10.1093/eurheartj/ehae021 PMid:38289867 PMCid:PMC10919918 |
||||
| 13.Vega-Cuéllar CI, Hurtado J, Aguirre J, de Dios-González J. Bloqueo de ganglio estrellado en el manejo de tormenta arrítmica en la miocardiopatía de Chagas. Revista Interameric Cardiol 2024; 1: doi: 10.24875/riac.23000017 https://doi.org/10.24875/RIAC.23000017 |
||||
| 14.Goncalves BKD, Sena MA, Silva W, Nascimento CEC do, Carrion JC. Electrical storms and Chagas disease: The role of epicardial ablation in long term follow up. J Am Coll Cardiol 2025; 85: 234. Doi: 10.1016/s0735-1097(25)00719-3 https://doi.org/10.1016/S0735-1097(25)00719-3 |
||||
| 15.Romero J, Velasco A, Pisani CF, Alviz I, Briceno D, Díaz JC, et al. Advanced therapies for ventricular arrhythmias in patients with Chagasic cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol 2021; 77: 1225-42. doi: 10.1016/j.jacc.2020.12.056 https://doi.org/10.1016/j.jacc.2020.12.056 PMid:33663741 |
||||
| 16. Pisani CF, Romero J, Lara S, Hardy C, Chokr M, Sacilotto L, et al. Efficacy and safety of combined endocardial/epicardial catheter ablation for ventricular tachycardia in Chagas disease: A randomized controlled study. Heart Rhythm 2020; 17: 1510-8. Doi: 10.1016/j.hrthm.2020.02.009 https://doi.org/10.1016/j.hrthm.2020.02.009 PMid:32087356 |
||||
| 17.Dabas, N, Cuervo J, Luna L, Colombo R. An uncommon common procedure: Ventricular aneurysmectomy in Chagas cardiomyopathy. J Am Coll Cardiol 2019; 73: 2960. doi: 10.1016/s0735-1097(19)33566-1 https://doi.org/10.1016/S0735-1097(19)33566-1 |
||||
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

