Factors associated with the development of stroke-associated pneumonia in Bishkek: An observational study
ORIGINAL RESEARCH ARTICLE
Factors associated with the development of stroke-associated pneumonia in Bishkek: An observational study
Article Summary
- DOI: 10.24969/hvt.2025.609
- CARDIOVASCULAR DISEASES
- Published: 24/11/2025
- Received: 27/09/2025
- Revised: 20/10/2025
- Accepted: 20/10/2025
- Views: 660
- Downloads: 402
- Keywords: Cerebral stroke, stroke – associated pneumonia, risk factors, predictors, multiple regression analysis, diagnostic accuracy
Address for Correspondence: Elmira M. Mamytova, I.K. Akhunbaev Kyrgyz State Medical Academy, Bishkek, Kyrgyzstan
Email: elmiramamytova@yahoo.com
ORCID: Turat S. Kadyrov - 0000-0003-4670-242Х; Elmira M. Mamytova - 0000-0002-4322-5555; A.Ch. Akunov -0000-0002-8176-0890; Aycholpon T. Israilova - 0009-0004-7373-5814; Aina Dj. Mamytova – 0009-0007-1637-6984,
Damirbek А. Abibillaev -0000-0002-4660-3064; Daniil A.О -0009-0003-4533-8937; Aijanat Nurbek k. -0009-0008-0488-249X
Turat S. Kadyrov1, Elmira M. Mamytova2, A.Ch. Akunov3, Damirbek А. Abibillaev3, Aycholpon T. Israilova2,
Aina Dj. Mamytova4, Daniil A. О2, Aijanat Nurbek k.5
1Bishkek City Clinical Multiprofile Hospital No. 2, Bishkek, Kyrgyzstan
2I.K. Akhunbaev Kyrgyz State Medical Academy, Bishkek, Kyrgyzstan
3 Ala-Too International University, Bishkek, Kyrgyzstan
4Kyrgyz State Medical Institute for Retraining and Further Professional Education, Bishkek, Kyrgyzstan
5Kyrgyz-Russian Slavic University, Bishkek, Kyrgyzstan
Abstract
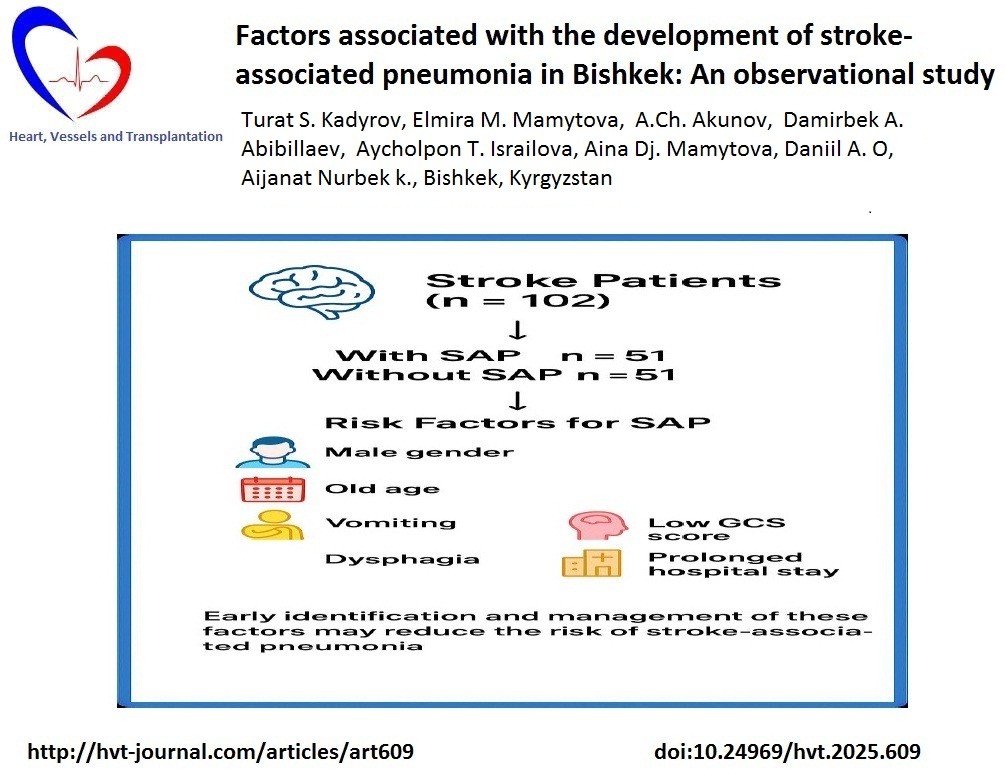
Objective: We aimed to verify the predictors of stroke- associated pneumonia (SAP) among hospitalized stroke patients in the conditions of the stroke department of an urban multidisciplinary hospital
Methods: The study design is a single–center, prospective, observational, cohort study on predictors of SAP in stroke patients and their diagnostic value. The study included 108 patients with stroke who received in-patient treatment from the Stroke Department in Bishkek City Clinical Multiprofile Hospital No. 2 and were divided into 2 groups: the main group consisted of 51 patients with SAP and the control group consisted of 57 patients without SAP. Demographic, clinical, neurological status, laboratory tests and neuroimaging parameters were analyzed to compose a predictive model with the determination of independent factors of SAP development.
Results: According to the results of multiple logistic regression analysis, it was revealed that the levels of systolic blood pressure, the presence of dysphagia, low Glasgow scale scores, age, as well as the level of INR at admission were independent factors associated with the development of SAP. The model showed a very good discrimination ability (AUC= 0.967, 95%CI 0.936-0.998p<0.001).
Conclusion: All three scales for assessing neurological deficits (Glasgow, NIHSS, and Rankin) differed statistically between the groups, however, it was the low Glasgow scale that was determined as an independent predictor of SAP. In addition, in our study, other independent predictors were high blood pressure, dysphagia, older age, and high INR.
Key words: Cerebral stroke, stroke – associated pneumonia, risk factors, predictors, multiple regression analysis, diagnostic accuracy

![]()
Graphical abstract
Introduction
Stroke is one of the leading causes of disability and mortality in many countries around the world. The burden of stroke is high and continues to rise in developing countries due to socio-demographic and lifestyle changes. Two thirds of all stroke cases and 80-85% patients have stroke-related disabilities and deaths occur in low- and middle-income countries (1-3). The outcome of a stroke mainly depends on the presence and severity of complications after a stroke (4).
Stroke-associated pneumonia (SAP) is pneumonia that occurs within 7 days after hospitalization in stroke patients who are not on mechanical ventilation (5). The incidence of SAP ranges from 7 to 38% according to various data, and mortality reaches 30-40% (6-10). This complication significantly affects the prognosis of patients, lengthens the period of hospitalization and increases the burden on the healthcare system (11).
Studies conducted in hospitals have shown that there are neurological and medical conditions that predispose to a higher incidence of SAP. Neurological risk factors include impaired consciousness, severe neurological deficits, higher NIHSS score, large stroke size, middle cerebral artery stroke, previous stroke, dysphagia, dysarthria/aphasia, and cranial nerve paralysis (9, 12-14). Medical risk factors include old age, male gender, poor functional condition, atrial fibrillation, anemia, hypoalbuminemia, hyperglycemia, endotracheal intubation, nasogastric tube feeding, and concomitant diseases such as heart failure, diabetes mellitus, and chronic lung diseases (9, 10, 12, 15, 16).
There is a lack of sufficient data on the frequency and predictors of SAP among stroke patients in Central Asian countries. Thus, the aim of this study is to try to fill this information gap and identify predictors of SAP among hospitalized stroke patients in this region.
Methods
Study design and population
The study design is a single-center, prospective, observational, cohort study on predictors of SAP in stroke patients and their diagnostic value.
The study included 102 patients of 504 patients with acute cerebral stroke who were admitted to the Stroke Department in the period of time from January 1, 2023 to December 31, 2023, who received in-patient treatment at the Stroke Department of Bishkek City Clinical Multiprofile Hospital No. 2.
Inclusion criteria: acute focal neurological deficit in combination with neuroimaging signs of cerebral infarction or intracerebral hematoma (excluding patients with subarachnoid hemorrhage), hospitalization within 72 hours after the onset of stroke symptoms, absence of infection for 2 weeks prior to admission and patients who gave informed consent for participation in the study. Exclusion criteria: transient ischemic attack, severe liver dysfunction, antibiotic treatment at the time of admission.
All the subjects were divided into 2 groups: the main group consisted of 51 patients with SAP and the control group consisted of 51 patients without SAP. All patients were matched by gender.
The research protocol was approved by the local Ethics committee of the I.K. Akhunbaev KSMA on 05/27/2023. This study was conducted according to Helsinki declaration 2024 standards were followed for care of patients and their informed consent was taken.
Clinical evaluation
Upon admission, a certified neurologist comprehensively determined the patient's pre-stroke infections based on the results of blood tests in the department, as well as the presence of symptoms of respiratory infection (cough, sputum, shortness of breath, fever, etc.) before the onset of stroke symptoms. In patients without pre-stroke infections, a certified neurologist was responsible for detailed registration of demographic and clinical data, including age, sex, time of onset of stroke symptoms, smoking status, alcoholism, medication intake, medical history (hypertension, diabetes mellitus, atrial fibrillation or coronary artery disease (CAD), chronic obstructive pulmonary disease (COPD), hyperlipidemia), and also, an assessment of the patient's condition related to stroke.
The initial severity of the stroke was assessed by a board-certified neurologist using the Stroke Scale of the National Institutes of Health (NIHSS) (17). Dysphagia was assessed using the Water swallowing Test (WST) (18). If the score of the water swallowing test was > 2, this result was taken as dysphagia.
The state of neurological function before the onset of stroke was assessed using the modified Rankin Scale (19). During the first 24 hours after admission, fasting venous blood was taken from each patient for routine laboratory measurements, including fasting glucose, serum liver enzymes, total cholesterol, serum creatinine, activated partial thromboplastin time (APTT), ptothrombin index (PTI) and international normalized ratio (INR), etc.
Upon admission, each patient underwent examination and treatment by certified neurologists in accordance with national guidelines for the treatment of acute cerebral stroke at the hospital level. In addition, for 7 -10 days after the onset of stroke symptoms, all patients had their body temperature and respiratory symptoms recorded daily after admission, a physical examination was performed, and the results of additional studies were monitored.
Stroke-associated pneumonia
SAP was defined as a spectrum of lower respiratory tract infections that developed during the first 7 days after the onset of stroke (20). In addition, this spectrum was taken into account in the presence of fever (> 38 ° C) and/or leukopenia (< 4000 × 109 cells / l) or leukocytosis (> 12000 × 109 cells / L), and at least two of the following criteria: (1) a new appearance of purulent sputum, a change in the nature of sputum or an increase in respiratory secretions, or an increase in the need for suction of secretions of the tracheo-bronchial tree; (2) a new appearance or intensification of coughing or shortness of breath or tachypnea; (3) crepitation or bronchial respiratory noises; (4) deterioration of gas exchange, increased oxygen demand.
In addition, SAP was diagnosed in the presence of additional typical changes on a chest X-ray or computed tomography (CT) (20, 21). The diagnosis of each case of acute stroke was confirmed by at least two attending physicians using diagnostic criteria for SAP
Study variables
The following variables were included in the analysis: sex, age, ethnicity, body mass index (BMI), vital signs such as systolic and diastolic blood pressure, respiratory rate, heart rate, oxygen saturation, temperature on admission to hospital; hospitalization duration; need in oxygen; risk factors – smoking and alcohol overuse; comorbidities – diabetes, CAD, COPD, atrial fibrillation; type of stroke – ischemic or hemorrhagic, neurological signs – dysphagia, neck stiffness, vomiting; neurological assessment scales - Glasgow Coma Scale (Glasgow coma Scale scores: with 15 being a fully awake and responsive state and 3 indicating a comatose or vegetative state) (22), NIHSS Scale (17), Rankin scale (19); hematology parameters – hemoglobin, leucocytes, platelets, lymphocytes, erythrocyte sedimentation rate (ESR), INR, and biochemical parameters – blood glucose, creatinine, bilirubin.
Statistical analysis
Statistical analysis was performed using the SPSS version 27 software (IBM, USA). For quantitative indicators, mean and values and standard deviations were calculated, and qualitative indicators were presented as the frequency (in n (%)). When comparing the two quantitative indicators, the Student's unpaired t-test was used. The Pearson agreement criterion (Chi-square test) was used in the qualitative analysis or the exact Fischer criterion. The differences were accepted as significant at p< 0.05.
Logistic regression was used to identify possible predictors of SAP development. Initially, the univariate analysis was performed for all indicators (sex, age, clinical, hematological, biochemical, neurological, including scales). Further, all indicators with a value of α<0.15 were selected for multiple logistic regression to reveal independent predictors of SAP development. The multiple logistic analysis with stepwise exclusion of indicators was used. Predictors considered independent and statistically significant at a level of α<0.05 were then included in the final prognostic model.
Next to determine diagnostic accuracy of model to predict SAP, the ROC analysis was performed and area under the curve (AUC) curve was constructed, sensitivity and specificity estimated.
Results
Among 504 patients admitted with acute cerebral stroke to the department during study period, 102 were eligible to be included in the study (Fig. 1) All the subjects were divided into 2 groups: the main group consisted of 51 patients with SAP and the control group consisted of 51 patients without SAP.
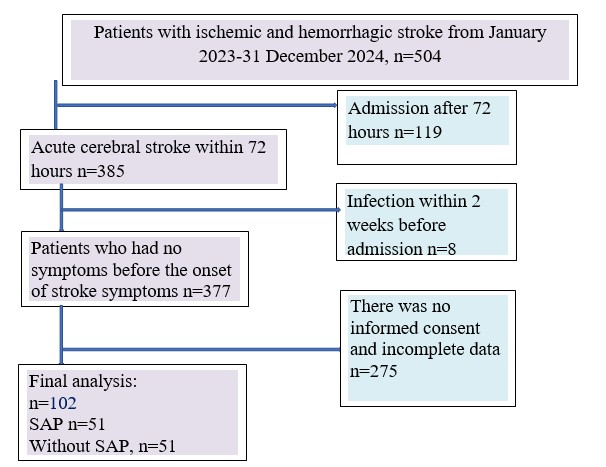
Figure 1. Flowchart of study patients recruitment according to STROBE guidelines
Clinical characteristics
The main demographic indicators are presented in Table 1. Patients with SAP were statistically significantly older than patients without SAP (p=0.04). At the same time, there were no significant differences between the groups in terms of BMI, male sex and Kyrgyz nationality.
All indicators of vital functions differed statistically when comparing the groups. Thus, systolic blood pressure in the SAP group was higher than in patients in the group without SAP (p =0.028) and exceeded normal values, while diastolic blood pressure, heart rate, respiratory rate, although were higher in the SAP group (all p<0.05), remained at the upper limit of the normal values. It is noteworthy that the oxygen saturation in the SAP group was closer to critical values (p<0.001), and the temperature was elevated, although it remained within subfebrile values (p<0.001) as compared to group without SAP.
Smoking and alcohol abuse were more common in patients in the main group (p<001 and p=0.044, respectively). There was also a high incidence of CAD and COPD in patients with SAP as compared to patients without (p<0.001, p=0.004). At the same time, there were no significant differences frequency of diabetes mellitus and atrial fibrillation (p>0.05).
From hematological parameters, at admission, higher leukocyte levels were observed in the main group compared with patients in the control group (p=0.006). In contrast, the levels of lymphocytes and platelets were statistically lower than in the control group (p <0.001 and p=0.005, respectively). There were no significant differences between the groups from the biochemical analyses parameters (p>0.05 for all). However INR was higher SAP group (p=0.03).
|
Table 1. Comparison of demographic, vital functions, comorbidities, hematological and biochemical variables between study groups |
|||
|
Variables |
SAP (n=51) |
Without SAP ( n=51) |
p* |
|
Demographic factors |
|||
|
Age, years |
69.8 (10.7) |
65.6 (9.6) |
0.04 |
|
Body mass index, kg/m2 |
26.6 (5.0) |
26.2 (3.4) |
0.69 |
|
Men, n (%) |
26 (51) |
26 (51) |
1.0 |
|
Kyrgyz ethnicity, n(%) |
35 (68.6) |
37 (72.5) |
0.67 |
|
Vital signs |
|||
|
Systolic BP, ммHg |
162 (36) |
148 (30) |
0.028 |
|
Diastolic BP, ммHg |
93 (13) |
86 (15) |
0.01 |
|
HR, per min |
87.2 (17.1) |
77.0 (10.8) |
<0.001 |
|
RR, per min |
18.8 (1.9) |
18.2 (0.7) |
0.022 |
|
SpO2, % |
91.7 (4.9) |
95.5 (2.9) |
<0.001 |
|
Temperature, С0 |
37.8 (0.9) |
36.4 (0.23) |
<0.001 |
|
Risk factors and comorbid conditions |
|||
|
Smoking, n(%) |
19 (37.3) |
1 (2.0) |
<0.001 |
|
Alcohol overusing, n(%) |
4 (7.8) |
0 (0) |
0.044 |
|
Diabetes mellitus, n(%) |
8 (15.7) |
13 (25.5) |
0.225 |
|
CAD, n(%) |
29 (56.9) |
12 (23.5) |
<0.001 |
|
COPD, n(%) |
12 (23.5) |
2 (3.9) |
0.004 |
|
Atrial fibrillation, n() |
7 (13.7) |
4 (7.8) |
0.343 |
|
Hematological and biochemical parameters |
|||
|
Hemoglobin, g/l |
134.4 (21.6) |
139.8 (21.9 |
0.215 |
|
Leucocytes, 109 |
10.98 (5.14) |
8.54 (3.51) |
0.006 |
|
Lymphocytes, |
13.53 (6.99) |
20.85 (9.98) |
<0.001 |
|
Platelets, 103 |
224 (60) |
269 (92) |
0.005 |
|
ESR, mm/h |
22.08 (15.50) |
17.53 (10.60) |
0.089 |
|
Glucose, mmol/l |
6.55 (2.51) |
6.89 (2.41) |
0.495 |
|
Creatinine, mmol/l |
94.4 (38.1) |
111.1 (47.8) |
0.07 |
|
Total bilirubin, mmol/l |
17.1 (12.1) |
15.0 (8.9) |
0.395 |
|
INR |
1.09 (0.15) |
1.03 (0.10 |
0.03 |
|
Data are presented as mean (SD) and n(%) *unpaired t test for independent samples, Chi-square or Fischer exact test BP – blood pressure, CAD – coronary artery disease, COPD – chronic obstructive pulmonary disease, ESR - erythrocytes sedimentation rate, HR – heart rate, INR - International normalized ratio RR-respiration rate, SAP – stroke associated pneumonia, SpO2 – oxygen saturation |
|||
Figure 2 shows the comparative length of stay and the frequency of oxygen therapy needs. Patients with SAP (the main group) stayed in the hospital longer (13.3 (4.2) vs. 9.3 (2.4) days, p <0.001) and needed oxygen therapy more often (82.4 vs. 21.6%, p<0.001).
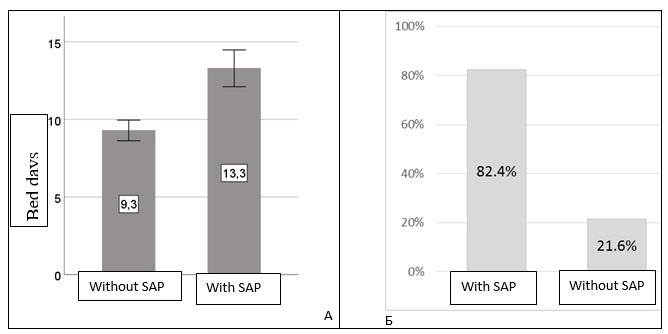
Figure 2. Duration of hospitalization (A) and frequency of oxygen support (B) in both groups
SAP – stroke-associated pneumonia
Table 2 shows the results of a comparison of neurological assessment scales, as well as the frequency of ischemic stroke and a number of neurological symptoms. Significant differences were found in all three scales, showing a more severe initial condition of patients in the main group (all p<0.001). There was also a significantly higher neck muscle rigidity and dysphagia in the study group compared with the control group (p<0.001 for both). However, there were no differences in the incidence of ischemic stroke.
|
Table 2. Comparison of neurological indicators and scales between study groups
|
|||
|
Variables |
SAP (n=51) |
Without SAP ( n=51) |
p* |
|
Glasgow coma Scale, score |
10.04 (2.27) |
14.12(1.69) |
<0.001 |
|
NIHSS Scale, score |
17.61 (7.14) |
6.71 (6.40) |
<0.001 |
|
Rankin Scale, score |
3.96 (0.75) |
2.12 (1.07) |
<0.001 |
|
Ischemic stroke, n(%) |
38 (74.5) |
45 (88.2) |
0.076 |
|
Vomiting, n(%) |
8 (15.7) |
5 (9.8) |
0.378 |
|
Neck stiffness, n(%) |
19 (37.3) |
4 (7.8) |
<0.001 |
|
Dysphagia, n(%) |
44 (86.3) |
4 (7.8) |
<0.001 |
|
Data are presented as mean (SD) and n(%) *unpaired t test for independent samples, Chi-square or Fischer exact test SAP – stroke associated pneumonia Interpretation of Glasgow coma Scale scores: with 15 being a fully awake and responsive state and 3 indicating a comatose or vegetative state. Interpretation of NIHSS - National Institutes of Health Stroke Scale scores: 0 is no stroke, 1-4 is a minor stroke, 5-15 is moderate, 16-20 is moderate-to-severe, and 21-42 is severe. Interpretation of Rankin Scale a scores: 1 indicates no significant disability, while higher scores indicate increasing levels of impairment, from slight disability (2) to severe disability requiring constant care (5) |
|||
According to the results of multiple logistic analysis (Table. 3) it was revealed that the level of systolic pressure, the presence of dysphagia, the Glasgow scale, age, as well as the level of INR upon admission are independent factors associated with the development of SAP.
|
Table 3. Results of multiple logistic analysis to identify independent predictors of SAP |
||||
|
Parameters |
β |
OR |
95 CI |
p |
|
Systolic BP |
0.033 |
1.034 |
1.008 – 1.061 |
0.011 |
|
Dysphagia |
3.785 |
44.053 |
6.463 – 300.291 |
<0.001 |
|
Glasgow coma Scale |
-0.557 |
0.573 |
0.393 – 0.834 |
0.004 |
|
Age |
0.078 |
1.081 |
1.003 – 1.165 |
0.041 |
|
INR |
0.915 |
2.496 |
1.077-5.784 |
0.033 |
|
Constant |
-14.849 |
0.0 |
|
<0.001 |
|
BP- blood pressure, CI – confidence interval, INR – international normalized ratio, OR – odds ratio, SAP – stroke associated pneumonia |
||||
Subsequently, we assigned the variables that entered the model. The assignment table was shown in Table 4. The regression model was: Logit (P) = −14.849+0.033X1+3.785X2 −0.557X3+0.078X4+0.915X5.
|
Table 4. Variable assignments |
||
|
Predictor |
Variable |
Remarks |
|
Systolic BP, mmHg |
X1 |
Continuous |
|
Dysphagia |
X2 |
0 = “No”, 1 = “Yes” |
|
Glasgow coma Scale, points |
X3 |
Continuous |
|
Age, years |
X4 |
Continuous |
|
INR* |
X5 |
Continuous |
|
BP- blood pressure, INR – international normalized ratio, * - actual INR should be divided in 10 |
||
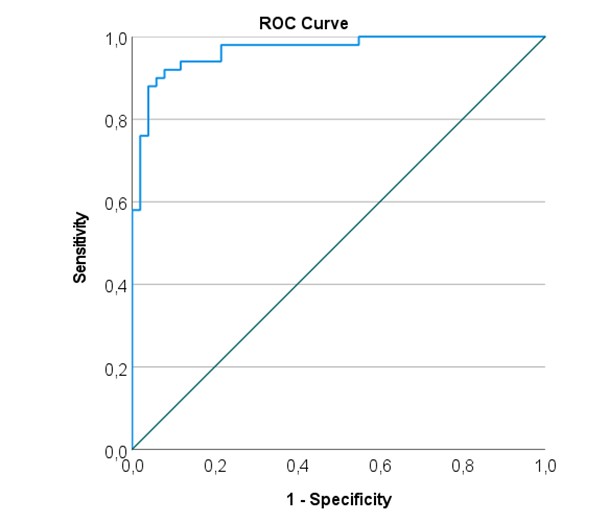
Figure 3 shows the ROC analysis curve constructed using the predictive model of multiple logistic analysis. It can be seen that the model shows a very good discrimination ability (AUC= 0.967, 95CI 0.936 to 0.998). The Youden index for the model was 0.842, with the cutoff of the probability (P) equaled to 0.472. Having this level of probability, the model showed a sensitivity of 92.0 and a specificity of 92.2 for SAP prediction.
![]()
|
Table 5. Diagnostic value of SAP predictors individually and in the general model |
|||
|
Predictor |
AUC |
95%CI |
p |
|
Systolic BP |
0.624 |
0.514 – 0.734 |
0.027 |
|
Dysphagia |
0.892 |
0.822 – 0.962 |
<0.001 |
|
15-GCS* |
0.915 |
0.853- 0.978 |
0.032 |
|
Age |
0.617 |
0.506 – 0.727 |
0.038 |
|
INR |
0.654 |
0.546- 0.763 |
0.005 |
|
Model |
0.967 |
0.936 – 0.998 |
<0.001 |
|
AUC – area under curve, BP- blood pressure, CI – confidence interval, GCS – Glasgo coma scale, INR – international normalized ratio, * Since the Glasgow scale score is the protective factor, the decrease from the maximum scores was used to calculate AUC. |
|||
Discussion
Pneumonia is a common type of infection after a stroke and significantly delays the recovering of neurological function (23).
All patients included in our study were hospitalized within 72 hours of the onset of the disease. The proportion of patients admitted to the department within 24 hours of the onset of stroke was 80.65, and patients with pre-stroke pulmonary and other infections were excluded from the study.
The indicator of vital functions as increased blood pressure has shown its predicative significance in relation to an increased risk of developing SAP. Other researchers also pointed to the association of SAP development with increased blood pressure, but this association mostly concerned severe hypertension (blood pressure above 200/120 mmHg), whereas in our study, the average blood pressure values did not reach 170 and 100 mmHg (24, 25).
The average saturation values are close to critical figures in patients with SAP, which, in our opinion, is a reflection of the severity of the neurological deficit and the level of impaired consciousness.
All three scales for assessing neurological deficits (Glasgow, NIHSS, and Rankin) differed statistically between the groups, however, it was the Glasgow scale that was determined as an independent predictor of SAP.
In our study, fever in patients with SAP was accompanied by leukocytosis and lymphopenia in the first days of admission. In one retrospective study, the SAP identifies the neutrophil-lymphocyte ratio as an independent risk factor and finds its association with an increased risk of acute apnea syndrome (26).
In our opinion, a more pronounced neurological deficit, dysphagia, and the need for oxygen therapy may be a favorable background for aspiration, which, combined with immunosuppression caused by stroke, contributes to the development of SAP through complex humoral and neural pathways that include the hypothalamic-pituitary-adrenal axis.
In addition to dysphagia, age and INR were other independent predictors in our study. Many studies have also pointed to a clear association of age and dysphagia with the risk of developing SAP (15, 22, 27-30).
A number of studies indicate that ischemic stroke and atrial fibrillation are also risk factors for the development of SAP (16, 22, 29); however, our study did not reveal such a relationship. This can be explained by the smaller number of observations in our case.
It may seem unexpected to identify the level of INR as a significant predictor of SAP development. However, in the last 2-3 years, several articles have appeared that draw attention to the relationship between INR and the development of SAP (31-34). The three of the four articles found higher INR levels in patients with SAP compared to control patients. Upon further analysis using multiple logistic regression, only one study (31) showed INR to be an independent predictor of SAP development. In the other two studies, D-dimer (32) and activated partial thromboplastin time (aPTT) (33) were independent predictors instead of INR, respectively. Eventually, it can be assumed that changes in the coagulation system, which involve INR, D-dimer, and aPTT, affect the likelihood of developing SAP. One possible explanation for this relationship may be that activation of coagulation in ischemic stroke leads to the consumption of coagulation factors and, consequently, to an increase in INR, D-dimer, as well as in a prolonged aPTT (30). As for the study, that found no differences in INR in patients with IAP (34), it included patients with ischemic stroke after the thrombolysis. This intervention (thrombolysis) could have significantly affected coagulation factors and, as a result, mitigate possible baseline differences in INR.
Study limitations
The results of this study cannot be extended to all stroke patients in Kyrgyzstan, as the study was conducted in one hospital. Another limitation of this study is the relatively small sample.
In addition, important predictors such as the patient's immune status and the time of nasogastric tube insertion were not included in this study due to the lack of documentation in the patient's medical records
Conclusion
The increased risk of pneumonia in patients with acute stroke can be predicted fairly accurately using a small set of clinical risk factors. High systolic blood pressure, older age, low score on the Glasgow Coma Scale, dysphagia, and high INR were risk factors that increased the risk of post-stroke pneumonia. Model including all these factors has high diagnostic accuracy in prediction of SAP.
Ethics: The research protocol was approved by the local Ethics committee of the I.K. Akhunbaev KSMA on 05/27/2023. This study was conducted according to Helsinki declaration 2024 standards for care of patients and their informed consent was taken.
Peer-review: External and internal
Conflict of interest: None to declare
Authorship: T.S.K., E.M.M., A.Ch. A., D.А. A., A.T.I.,
A.Dj.M., D.A.О., A.N.k. equally contributed to the study and manuscript preparation , approved the final manuscript and
fulfilled the authorship criteria
Acknowledgements and funding: None to declare
Statement on A.I.-assisted technologies use: Authors did not use A.I. technology in preparation of manuscript
Data and material availability: Contact authors. Any share should in frame of fair use with acknowledgement of source and/or collaboration.
References
| 1.Feigin VL, Norrving B, Mensah GA.Global burden of stroke. Circ Rese 2017; 120: 439-48. doi: 10.1161/circresaha.116.308413 https://doi.org/10.1161/CIRCRESAHA.116.308413 PMid:28154096 |
||||
| 2.Johnson W, Onuma O, Owolabi M, Sachdev S. Stroke: A global response is needed. Bulletin of the World Health Organization 2016; 94, 634-634A. doi:10.2471/blt.16.181636 https://doi.org/10.2471/BLT.16.181636 PMid:27708464 PMCid:PMC5034645 |
||||
| 3.Owolabi M, Akarolo-Anthony S, Akinyemi R, Arnett D, Gebregziabher M, Jenkins C, et al. (2015). The burden of stroke in Africa: A glance at the present and a glimpse into the future: Review article. Cardiovasc J Africa 2015; 26; S27-S38. doi:10.5830/cvja-2015-038 https://doi.org/10.5830/CVJA-2015-038 PMid:25962945 PMCid:PMC4557491 |
||||
| 4.Adeloye D. An estimate of the incidence and prevalence of stroke in Africa: A systematic review and meta-analysis. PLoS ONE 2014; 9, e100724. Doi: 10.1371/journal.pone.0100724 https://doi.org/10.1371/journal.pone.0100724 PMid:24967899 PMCid:PMC4072632 |
||||
| 5.Ji R, Wang D, Shen H, Pan Y, Liu G, Wang P, et al. Interrelationship among common medical complications after acute stroke. Stroke 2013; 44: 3436-444. Doi: 10.1161/strokeaha.113.001931 https://doi.org/10.1161/STROKEAHA.113.001931 PMid:24178914 |
||||
| 6.Gittins M, Lobo Chaves MA, Vail A, Smith C J. Does stroke-associated pneumonia play an important role on risk of in-hospital mortality associated with severe stroke? A four-way decomposition analysis of a national cohort of stroke patients. Int J Stroke 2023; 18: 1092-101. Doi: 10.1177/17474930231177881 https://doi.org/10.1177/17474930231177881 PMid:37170807 PMCid:PMC10614175 |
||||
| 7.Teh WH, Smith CJ, Barlas RS, Wood AD, Bettencourt-Silva JH, Clark AB, et al. Impact of stroke-associated pneumonia on mortality, length of hospitalization, and functional outcome. Acta Neurol Scand 2018; 138: 293-300. doi: 10.1111/ane.12956 https://doi.org/10.1111/ane.12956 PMid:29749062 |
||||
| 8.Yu Y-J, Weng W-C, Su F-C, Peng T-I, Chien Y-Y, Wu C-L, et al. Association between pneumonia in acute stroke stage and 3-year mortality in patients with acute first-ever ischemic stroke. J Clin Neurosci 2016; 33: 124-8. doi: 10.1016/j.jocn.2016.02.039 https://doi.org/10.1016/j.jocn.2016.02.039 PMid:27436765 |
||||
| 9.Matz K, Seyfang L, Dachenhausen A, Teuschl Y, Tuomilehto J, Brainin M. Post-stroke pneumonia at the stroke unit - a registry based analysis of contributing and protective factors. BMC Neurol 2016; 16: doi: 10.1186/s12883-016-0627-y https://doi.org/10.1186/s12883-016-0627-y PMid:27430328 PMCid:PMC4949772 |
||||
| 10.Vyas L, Kulshreshtha D, Maurya P, Singh A, Qavi A, Thacker A. A2DS2 score to predict the risk of stroke-associated pneumonia in acute stroke: An Indian perspective. J Neurosci Rural Pract 2019; 10: 465-71. doi: 10.1055/s-0039-1697893 https://doi.org/10.1055/s-0039-1697893 PMid:31595119 PMCid:PMC6779542 |
||||
| 11.Koennecke H-C, Belz W, Berfelde D, Endres M, Fitzek S, Hamilton F, et al. Factors influencing in-hospital mortality and morbidity in patients treated on a stroke unit. Neurol 2011; 77: 965-72. doi: 10.1212/wnl.0b013e31822dc795 https://doi.org/10.1212/WNL.0b013e31822dc795 PMid:21865573 |
||||
| 12.Sellars C, Bowie L, Bagg J, Sweeney MP, Miller H, Tilston J, Stott DJ. Risk factors for chest infection in acute stroke. Stroke 2007; 38: 2284-91. Doi: 10.1161/strokeaha.106.478156 https://doi.org/10.1161/STROKEAHA.106.478156 PMid:17569875 |
||||
| 13.Russell JBW, Charles E, Conteh V, Lisk DR. Risk factors, clinical outcomes and predictors of stroke mortality in Sierra Leoneans: A retrospective hospital cohort study. Ann Med Surg 2020; 60: 293-300. doi:10.1016/j.amsu.2020.10.060 https://doi.org/10.1016/j.amsu.2020.10.060 PMid:33204420 PMCid:PMC7649580 |
||||
| 14.Mohammed AS, Degu A, Woldekidan NA, Adem F, Edessa D. In-hospital mortality and its predictors among stroke patients in sub-Saharan Africa: A systemic review and meta-analysis. SAGE Open Med 2021; 9: doi:10.1177/20503121211036789 https://doi.org/10.1177/20503121211036789 PMid:34377477 PMCid:PMC8326621 |
||||
| 15.Finlayson O, Kapral M, Hall R, Asllani E, Selchen D, Saposnik G. Risk factors, inpatient care, and outcomes of pneumonia after ischemic stroke. Neurol 2011; 77: 1338-45. Doi: 10.1212/wnl.0b013e31823152b1 https://doi.org/10.1212/WNL.0b013e31823152b1 PMid:21940613 |
||||
| Heart, Vessels and Transplantation 2025; 9: doi: 10.24969/hvt.2025.609 | ||||
| Predictors of SAP development Kadyrov et al. | ||||
| 16.Hoffmann S, Malzahn U, Harms H, Koennecke H-C, Berger K, Kalic M. Development of a clinical score (A2DS2) to predict pneumonia in acute ischemic stroke. Stroke 2012; 43: 2617-23. Doi: 10.1161/strokeaha.112.653055 https://doi.org/10.1161/STROKEAHA.112.653055 PMid:22798325 |
||||
| 17.Chalos V, van der Ende NAM, Lingsma HF, Mulder M, Venema E, Dijkland SA, et al. National institutes of health stroke scale: an alternative primary outcome measure for trials of acute treatment for ischemic stroke. Stroke 2020; 51:282-90. doi: 10.1161/STROKEAHA.119.026791 https://doi.org/10.1161/STROKEAHA.119.026791 PMid:31795895 PMCid:PMC6924951 |
||||
| 18.Chen PC, Chuang CH, Leong CP, Guo SE, Hsin YJ. Systematic review and meta-analysis of the diagnostic accuracy of the water swallow test for screening aspiration in stroke patients. J Adv Nurs 2016; 72: 2575-86. doi: 10.1111/jan.13013 https://doi.org/10.1111/jan.13013 PMid:27237447 |
||||
| 19.Wang M, Rajan SS, Jacob AP, Singh N, Parker SA, Bowry R, et al. Retrospective collection of 90-day modified Rankin scale is accurate. Clin Trials 2020; 17: m637-43. doi: 10.1177/1740774520942466 https://doi.org/10.1177/1740774520942466 PMid:32755236 |
||||
| 20.Smith CJ, Kishore AK, Vail A, Chamorro A, Garau J, Hopkins SJ, et al. Diagnosis of stroke-associated pneumonia: recommendations from the pneumonia in stroke consensus group. Stroke 2015; 46: 2335-40. doi: 10.1161/STROKEAHA.115.009617 https://doi.org/10.1161/STROKEAHA.115.009617 PMid:26111886 |
||||
| 21.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 2008; 36:309-32. doi: 10.1016/j.ajic.2008.03.002 https://doi.org/10.1016/j.ajic.2008.03.002 PMid:18538699 |
||||
| 22.Hotter B, Hoffmann S, Ulm L, Meisel C, Bustamante A, Montaner, J, et al. External validation of five scores to predict stroke-associated pneumonia and the role of selected blood biomarkers. Stroke 2021; 52: 325-30. doi: 10.1161/strokeaha.120.031884 https://doi.org/10.1161/STROKEAHA.120.031884 PMid:33280547 |
||||
| 23.Hotter B, Hoffmann S, Ulm L, Montaner J, Bustamante A, Meisel C, et al. Inflammatory and stress markers predicting pneumonia, outcome, and etiology in patients with stroke. Neurol Neuroimmun & Neuroinfl 2020; 7: doi: 10.1212/nxi.0000000000000692 https://doi.org/10.1212/NXI.0000000000000692 PMid:32098866 PMCid:PMC7051196 |
||||
| 24.Hannawi Y, Hannawi B, Rao C, Suarez I, Bershad E, Stroke-associated pneumonia: Major advances and obstacles. Cerebrovasc Dis 2013; 35: 430-43. Doi: 10.1159/000350199 https://doi.org/10.1159/000350199 PMid:23735757 |
||||
| 25.Ishigami K, Okuro M, Koizumi Y. Association of severe hypertension with pneumonia in elderly patients with acute ischemic stroke. Hypertens Res 2012; 35: 648-53. Doi: 10.1038/hr.2012.7 https://doi.org/10.1038/hr.2012.7 PMid:22318204 PMCid:PMC3368232 |
||||
| 26.Quanpeng Wang, Yao Liu, Ling Han, Fei He, Nan Cai, Qiuling Zhang, Jun Wang, Risk factors for acute stroke-associated pneumonia and prediction of neutrophil-to-lymphocyte ratios. Am J Emerg Med Volume 2021; 41:55-9. 27.Chang MC, Choo YJ, Seo KC, Yang S. The relationship between dysphagia and pneumonia in acute stroke patients: A systematic review and meta-analysis. Front Neurol 2022; 13: doi:10.3389/fneur.2022.834240 https://doi.org/10.3389/fneur.2022.834240 PMid:35370927 PMCid:PMC8970315 |
||||
| 28.Martino R, Foley N, Bhogal S, Diamant N, Speechley M, Teasell R. Dysphagia after stroke. Stroke 2005; 36: , 2756-63. Doi: 10.1161/01.str.0000190056.76543.eb https://doi.org/10.1161/01.STR.0000190056.76543.eb PMid:16269630 |
||||
| 29.Kuo Y-W, Huang Y-C, Lee M, Lee T-H, Lee J-D. Risk stratification model for post-stroke pneumonia in patients with acute ischemic stroke. Eur J Cardiovasc Nurs 2019; 19: 513-20. Doi: 10.1177/1474515119889770 https://doi.org/10.1177/1474515119889770 PMid:31735079 |
||||
| 30.Xie X, Wang X, Li Z, Zhao X, Miao Z, Liu L, et al. Prognostic value of international normalized ratio in ischemic stroke patients without atrial fibrillation or anticoagulation therapy. J Atheroscl Thromb 2019; 26: 378-87. doi:10.5551/jat.43752 https://doi.org/10.5551/jat.43752 PMid:30318486 PMCid:PMC6456455 |
||||
| 31.Li Y, Zhao L, Liu Y, Lu Y, Yao J, Li C, et al. (2022). Novel predictors of stroke-associated pneumonia: A single center analysis. Front Neurol 2022; 13: doi: 10.3389/fneur.2022.857420 https://doi.org/10.3389/fneur.2022.857420 PMid:35432153 PMCid:PMC9007082 |
||||
| 32.Li D, Liu Y, Jia Y, Yu J, Chen X, Li H, et al. Evaluation of a novel scoring system based on thrombosis and inflammation for predicting stroke-associated pneumonia: A retrospective cohort study. Front Aging Neurosci 2023; 15: 1153770. doi: 10.3389/fnagi.2023.1153770 https://doi.org/10.3389/fnagi.2023.1153770 PMid:37065465 PMCid:PMC10098085 |
||||
| 33.Lin G, Hu M, Song J, Xu X, Liu H, Qiu L, et al. High fibrinogen to albumin ratio: A novel marker for risk of stroke-associated pneumonia? Front. Neurol 2022; 12:747118. doi: 10.3389/fneur.2021.747118 https://doi.org/10.3389/fneur.2021.747118 PMid:35095715 PMCid:PMC8792987 |
||||
| 34.Krongsut S, Soontornpun A, Anusasnee N. Serial ASPECTS to predict stroke-associated pneumonia after thrombolysis in patients with acute ischemic stroke. Front Neurol 2024; 15: 1364125. doi: 10.3389/fneur.2024.1364125 https://doi.org/10.3389/fneur.2024.1364125 PMid:38711555 PMCid:PMC11071176 |
||||
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

