A paradigm shift in emergency management for left ventricular assist device recipients
EDITORIALS
A paradigm shift in emergency management for left ventricular assist device recipients
Article Summary
- DOI: 10.24969/hvt.2025.569
- CARDIOVASCULAR DISEASES
- Published: 01/06/2025
- Received: 27/05/2025
- Accepted: 27/05/2025
- Views: 3172
- Downloads: 1803
- Keywords: Left ventricular assist devices, mangement, mechanical circulatory support, end-stage heart failure, transplantation, guidelines
Address for Correspondence: Salvatore Scianna, Department of Cardiovascular Surgery, Policlinico Morgagni Hospital, University of Kern, Pedara, Italy
Email: scianna.sal@gmail.com Mobile: +39 388 424 8380
ORCID: 0000-0002-1798-2157
Salvatore Scianna
Centro Cuore Morgagni Pedara, Kore University of Enna, Pedara, Enna, Sicily, Italy
Graphical abstract

Key words: Left ventricular assist devices, mangement, mechanical circulatory support, end-stage heart failure, transplantation, guidelines
The advent of implantable left ventricular assist devices (LVADs) has fundamentally transformed the therapeutic landscape for patients with end-stage heart failure, offering a crucial bridge to transplantation, myocardial recovery, or as definitive destination therapy (1). Concomitant with the expanding utilization of these sophisticated mechanical circulatory support (MCS) systems is the escalating imperative for specialized, evidence-informed protocols to manage life-threatening emergencies in this unique patient cohort. The recently promulgated "British societies guideline on the management of emergencies in implantable left ventricular assist device recipients in transplant centres" by Akhtar et al. (2) in Intensive Care Medicine represents a seminal contribution, articulating a standardized, pragmatic framework for urgent intervention within specialized centers.
LVAD recipients manifest a distinct physiological milieu. Contemporary continuous-flow LVADs generate non-pulsatile or minimally pulsatile systemic circulation, thereby confounding conventional hemodynamic assessment modalities. Non-invasive blood pressure measurement, pulse oximetry, and even palpable pulse detection can be unreliable or absent, demanding a nuanced clinical interpretative skillset (3). This "LVAD paradox" - whereby a patient may exhibit preserved consciousness despite hemodynamic parameters conventionally indicative of extremis—necessitates a departure from standard resuscitation algorithms. The intricate interplay between the MCS device and native cardiac function, compounded by inherent device-related complications such as hemorrhage, thrombosis, infection, and electromechanical malfunction, underscores the requirement for bespoke emergency management strategies (1, 4). Historically, a significant degree of clinical incertitude has pervaded the application of cardiopulmonary resuscitation (CPR) in LVAD patients. Apprehensions regarding cannula dislodgement, device damage, or disruption of anastomotic integrity often precipitated hesitancy or omission of chest compressions. The Akhtar et al. (2) guideline directly confronts this ambiguity, drawing upon emergent data suggesting that the iatrogenic risks associated with CPR may have been previously overestimated, particularly in the chronic post-implantation phase (5, 6). The central tenet and principal innovation of this guideline is its "pump-first" doctrine. It advocates for a circumscribed, maximal two-minute deferral of chest compressions to facilitate immediate attempts at restoring LVAD functionality. This recommendation is predicated on the pathophysiological understanding that a non-operational continuous-flow LVAD, lacking an outflow valve, can permit substantial retrograde aortic flow into the left ventricle. Such retrograde flow severely compromises systemic and cerebral perfusion, thereby attenuating the efficacy of external cardiac compressions (2). Consequently, re-establishing pump function is posited as the most physiologically potent resuscitative intervention.
The algorithm proffered by the Joint British Societies and Transplant Centres LVAD Working Group is characterized by its clarity, conciseness, and operationalizability, specifically designed for first responders within advanced heart failure centers. Upon encountering an unresponsive LVAD recipient, the initial action, subsequent to summoning expert assistance, is explicitly directed towards "CHECK IS LVAD WORKING?" (2). This directive reorients the initial resuscitative focus from the patient's chest to the LVAD controller interface.
The guideline systematically navigates responders through the diagnostic and therapeutic pathways for common LVAD alerts and critical scenarios(Fig. 1):
• Blank Controller: Addressing potential power depletion or controller failure.
• Driveline Disconnection: Mandating comprehensive inspection and reconnection of driveline integrity.
• Low/Critical Battery: Requiring immediate battery replacement or connection to a mains power source.
• High Power Consumption (Watts): Indicative of potential pump thrombosis, a critical emergency necessitating specialist consultation and potentially urgent interventions such as thrombolysis or device exchange (7).
• Low Flow Alarm: This frequent alarm initiates a diagnostic cascade encompassing hypovolemia (addressed via passive leg raise and judicious fluid administration), right ventricular failure (where fluid loading may be deleterious), or excessive afterload (systemic hypertension) (2, 8).
• Ventricular Arrhythmias (VT/VF): Recognizing that such arrhythmias may be hemodynamically tolerated due to sustained LVAD support, the guideline advocates defibrillation primarily in unresponsive patients, or synchronized cardioversion (with appropriate sedation if conscious) if circulatory compromise is evident (2).
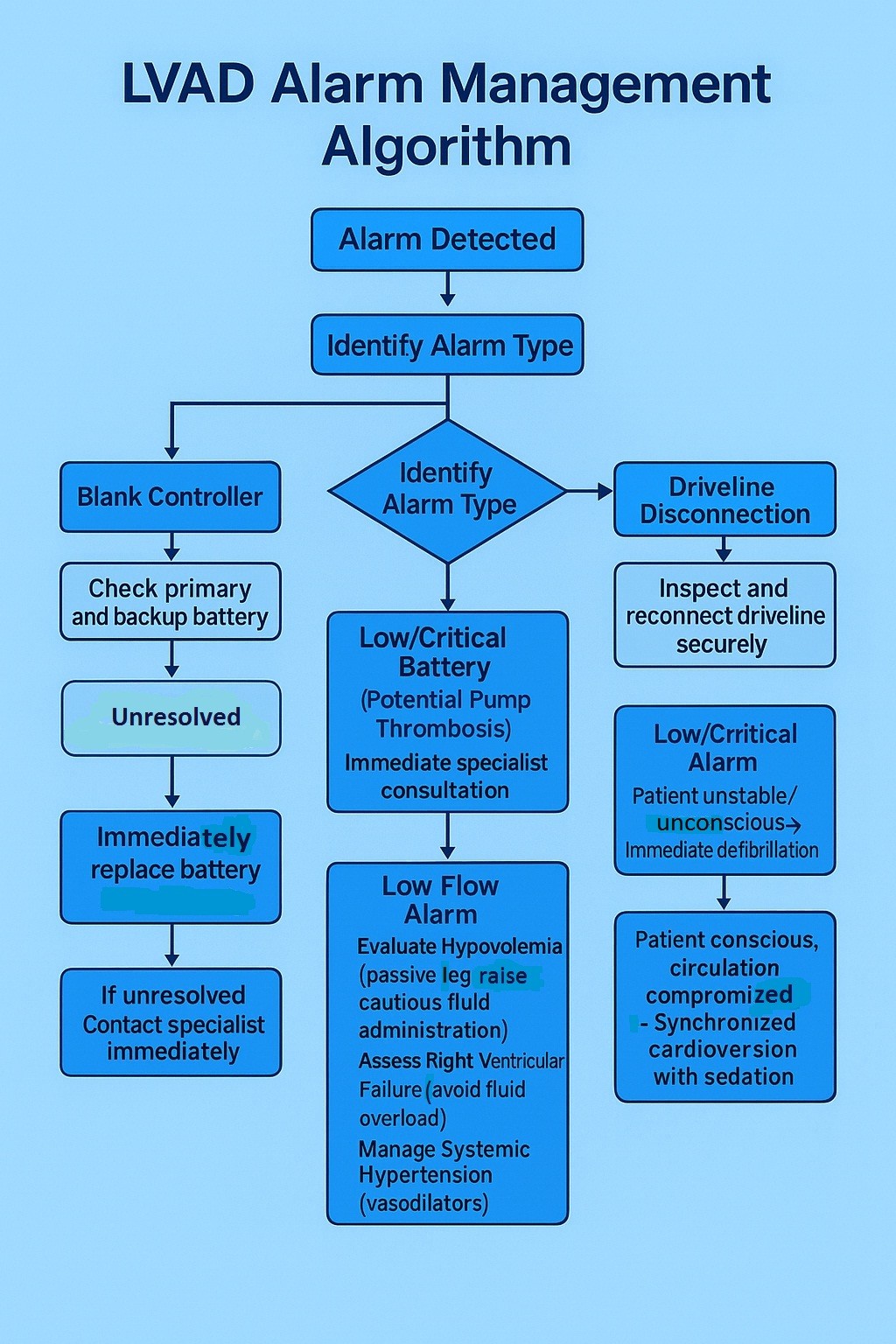
Figure 1. LVAD management flow chart
LVAD – left ventricular assisted device
Only subsequent to these device-specific evaluations and interventions, if circulatory inadequacy persists—as determined by responsiveness, central cyanosis, capillary refill time, Doppler-derived mean arterial pressure, audible LVAD hum/flow parameters, and end-tidal carbon dioxide levels—does the algorithm transition towards conventional resuscitative measures.
A critical differentiating factor is the temporal proximity to LVAD implantation: within 10 days post-operatively, emergent surgical re-exploration (re-sternotomy) is advocated; beyond this period, standard Advanced Life Support (ALS) protocols, including chest compressions, are initiated, alongside systematic consideration of the '4Hs and 4Ts' and potential escalation to temporary MCS, such as veno-arterial extracorporeal membrane oxygenation (VA-ECMO) (2, 9). The guideline judiciously incorporates echocardiography as an adjunctive diagnostic modality, when available, to evaluate for right ventricular dysfunction, suction events, pericardial tamponade, or intracardiac thrombus, while acknowledging the inability of echocardiography to visualize intra-pump thrombus.
The robustness of this guideline is attributable not only to its meticulously crafted content but also to its collaborative genesis, involving key UK transplant centers and pertinent national societies. This consensus-driven methodology fosters broad acceptance and facilitates standardized implementation. The explicit emphasis on structured, simulation-based training is paramount, as the effective deployment of such algorithms is contingent upon clinician proficiency and confidence (10). While specifically developed for the UK healthcare context, its core principles possess extensive applicability to any institution routinely managing LVAD recipients. Prospectively, while this guideline constitutes a significant advancement, the field remains inherently dynamic.
Further empirical research is warranted to refine our comprehension of CPR hemodynamics in the context of (non)-functional LVADs. The safety and efficacy of mechanical CPR devices within this patient demographic require more rigorous investigation (6). Furthermore, systematic collection of outcome data following the implementation of this algorithm will be indispensable for validating its clinical effectiveness and identifying avenues for future refinement. The guideline also implicitly acknowledges the sobering prognosis for LVAD patients requiring CPR (5), thereby accentuating the imperative for early recognition of clinical deterioration and timely intervention, but also underscoring the critical necessity for comprehensive advance care planning discussions with these patients and their families (11).
In summary, Akhtar et al. (2) have furnished an invaluable, evidence-informed resource for clinicians tasked with managing emergencies in LVAD recipients. By prioritizing rapid LVAD assessment and targeted troubleshooting prior to the reflexive initiation of standard resuscitation protocols, this guideline empowers frontline healthcare professionals to deliver more nuanced, physiologically rational, and potentially life-sustaining care. It signifies a crucial transition from clinical ambiguity to algorithmic clarity, ensuring that when the vital hum of the LVAD ceases, the ensuing response is swift, logical, and meticulously tailored to the unique pathophysiological exigencies of this complex patient population. This seminal work will undoubtedly elevate the standard of emergency care for LVAD recipients and serve as a foundational reference for future international guidance.
Peer-review: Internal
Conflict of interest- None to declare
Authorship: S.S.
Acknowledgement and Funding: None to declare
Statement on A.I.-assisted technologies use- Authors declare that they did not use AI-assisted technologies in preparation of this manuscript
Availability of data and material: Do not apply
References
| 1.Saeed D, Feldman D, El Banayosy A, et al. The 2023 International Society for Heart and Lung Transplantation guidelines for mechanical circulatory support: A 10-Year Update. J Heart Lung Transplant 2023; 42: e1-e122. | ||||
| 2.Akhtar W, Rial Baston V, Berman M, et al. British societies guideline on the management of emergencies in implantable left ventricular assist device recipients in transplant centres. Intensive Care Med 2024; 50: 493-501. https://doi.org/10.1007/s00134-024-07382-y PMid:38526578 PMCid:PMC11018667 |
||||
| 3.Lankheet S, Pieterse MM, Rijnhout R, et al. Validity and success rate of noninvasive mean arterial blood pressure measurements in cf-LVAD patients: a technical review. Artif Organs 2022; 46: 2361-70. https://doi.org/10.1111/aor.14367 PMid:35920238 PMCid:PMC9804858 |
||||
| 4.Truby L, Takeda K, Yuzefpolskaya M, et al. Bleeding and thrombosis in left ventricular assist device therapy. Circ Res 2022; 130:1198-215. | ||||
| 5.Barssoum K, Patel H, Rai D, et al. Outcomes of cardiac arrest and cardiopulmonary resuscitation in patients with left ventricular assist device: An insight from a National Inpatient Sample. Heart Lung Circ 2022; 31: 246-54. https://doi.org/10.1016/j.hlc.2021.05.096 PMid:34226105 |
||||
| 6.Shinar Z, Drozdova P, Stahovich M, et al. Chest compressions in left ventricular assist device patients: A systematic review and meta-analysis. ASAIO J 2023; 69: 525-30. | ||||
| 7.Kilic A, Hickey G, Mathier MA, et al. Clinical Presentation and outcomes of pump thrombosis in the HeartMate 3 LVAD: A single-center experience. ASAIO J 2022; 68: 54-9. | ||||
| 8.Kassis E, Uriel N, Sayer G, et al. Clinical presentation and management of right heart failure in patients with left ventricular assist devices. JACC Heart Fail 2021; 9: 777-89. | ||||
| 9.Peura JL, Rali AS, Lemor A, et al. Venoarterial extracorporeal membrane oxygenation in patients with left ventricular assist devices. ASAIO J 2020; 66: 11-6. | ||||
| 10.Akhtar W, Gamble B, Kiff K, et al. Mechanical life support algorithm developed by simulation for inpatient emergency management of recipients of implantable left ventricular assist devices. Resusc Plus 2022; 11: 100254. https://doi.org/10.1016/j.resplu.2022.100254 PMid:35669526 PMCid:PMC9162943 |
||||
| 11.McIlvennan CK, Allen LA. Advance care planning in patients with left ventricular assist devices: a review. JAMA Intern Med 2022; 182: 446-53. | ||||
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

