Insights from the EACVI document on multimodality imaging for the evaluation and management of patients with long-term (durable) left ventricular assist devices
EDITORIALS
Insights from the EACVI document on multimodality imaging for the evaluation and management of patients with long-term (durable) left ventricular assist devices
Article Summary
- DOI: 10.24969/hvt.2025.584
- CARDIOVASCULAR DISEASES
- Published: 20/08/2025
- Received: 11/08/2025
- Accepted: 11/08/2025
- Views: 2053
- Downloads: 933
- Keywords: Left ventricular assist devices, evaluation, imaging, echocardiography, cardiac magnetic resonance imaging, computed tomography, positron emission tomography/ computed tomography
Address for Correspondence: Aditya Sood, Conway Medical center, Conway, SC, USA
E-mail: aditya.sood@gmail.com ORCID: 0000-0002-4849-1879
Aditya Sood
Conway Medical center, Conway, SC, USA
Graphical abstract
Key words: Left ventricular assist devices, evaluation, imaging, echocardiography, cardiac magnetic resonance imaging, computed tomography, positron emission tomography/ computed tomography
The ever-evolving field of durable left ventricular assist devices (LVAD) continues to transform the realm of treatment options for patients with end-stage heart failure.
A Journal of American College of Cardiology scientific statement by Tedford et al (1) showed average
survival is now at par with that of heart transplantation at 2 years, with 5-year survival data approaching 58% for the third generation centrifugal pumps (HeartMate III) (2). Durable LVADs require significant evaluation in the preoperative, perioperative and postoperative phase for successful outcomes.
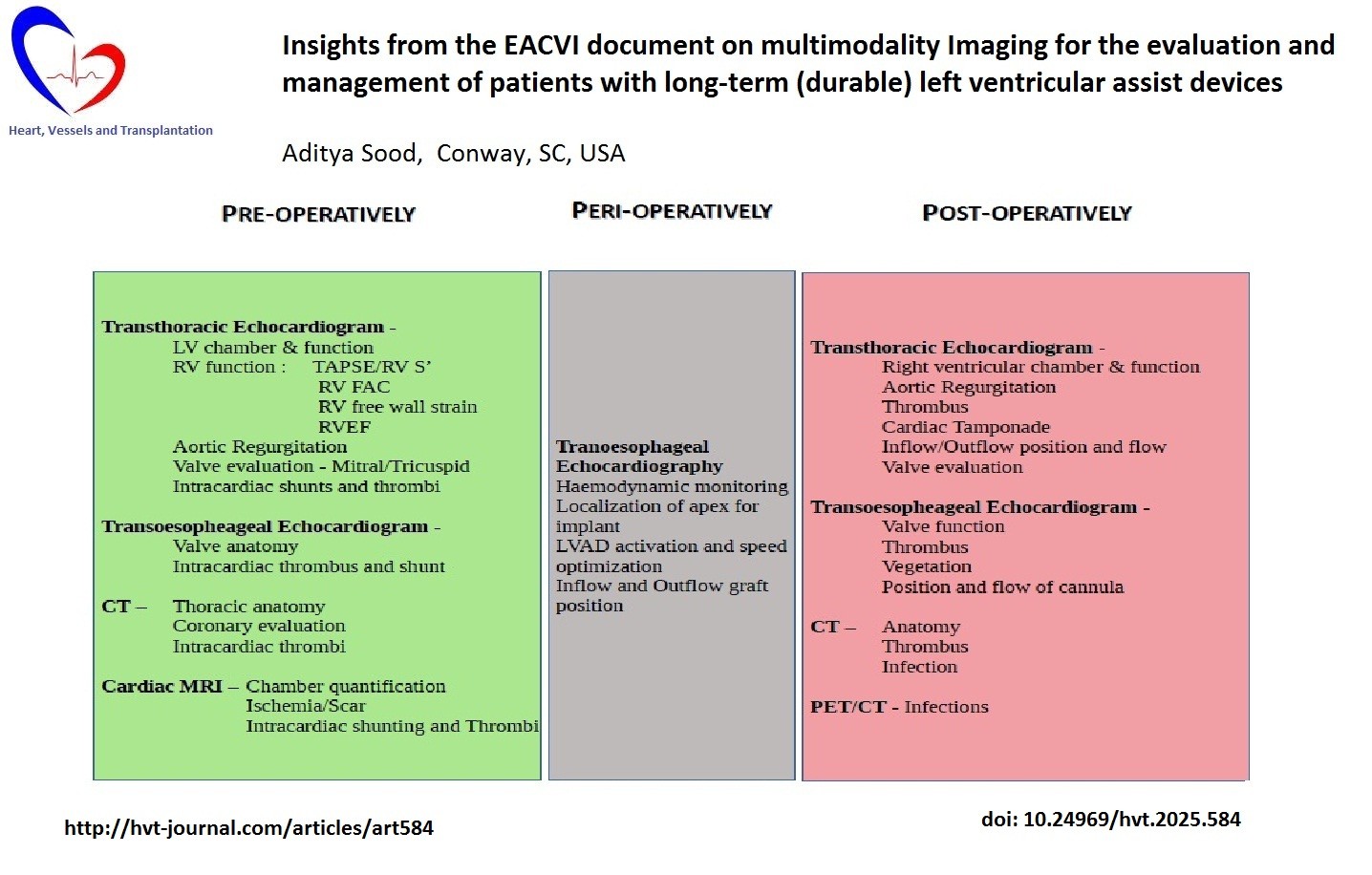
Echocardiography has traditionally served as the preferred non-invasive technique for evaluation of LVAD (3, 4), however with the increasing technological advances and applied artificial intelligence tools the role of multimodality imaging has expanded.
LVAD architecture and physiology is distinct for every device, thus posing tailored imaging challenges to assess function. Modern devices such as the HeartMate 3 are centrifugal pumps that continuously drain blood from the left ventricle delivering it into the aorta, generating minimally pulsatile systemic blood flow.
Echocardiography is crucial to LVAD imaging – from initial bi-ventricular assessment of function and patient selection for implantation (4) to identification of cannula position as well as long- term complications.
Cameli and colleagues` (5) recent clinical consensus document published in the European Heart Journal - Cardiovascular Imaging (November 2024) provides an in depth exhaustive review of the current state-of-affairs of cardiovascular imaging.
Right ventricular (RV) evaluation is paramount to successful LVAD implantation. Validated echo parameters studied include RV dimensions (basal, longitudinal and medium diameter) and function. The 2-dimensional (2D) RV parameters such as tricuspid annular plane systolic excursion (TAPSE), tissue Doppler derived tricuspid lateral annular systolic velocity wave (S’), RV fractional area change (RVFAC) have traditionally been employed to assess RV function (6).
Newer modalities employ 3-dimensional (3D) imaging tools; speckle tracking echocardiography and evolving software intelligence. RV global longitudinal strain (RV GLS) and free wall longitudinal strain (fwRVLS) are considered accurate measures of RV function. Meta-analysis data proves RV GLS and fwRVLS tracking accurately predict subsequent RV dysfunction after LVAD implantation. Furthermore, fwRVLS (<-22%) has good correlation with RV stroke volume index (7-8) and studies have shown fwRVS a reliable prognostic marker to predict early RV failure with a cut-off values > -14% (9). In essence, fwRVLS is considered the most sensitive marker prognostic marker for RV dysfunction post implantation (9).
The 3D imaging can circumvent the often complex RV geometry leading to inaccurate RVFAC evaluation (10). The 3D echocardiography (3DE) is the most accurate method to evaluate ventricular volumes and ejection fraction (11). The 3D technology is particularly useful in indeterminate situations, with research showing superior predictors of RV failure than conventional 2D. Applications of 3D and speckle technology have benefited from software improvement and integration such as auto tracking features enabling wider utilization in the community.
Transesophageal echocardiography (TOE) is indispensable in the intraoperative and perioperative phases as delved into detail in this consensus statement.
TOE aids in comprehensive valve, intracardiac shunting and thrombus evaluation. TOE is a vital tool for hemodynamic monitoring during LVAD implantation. TOE is utilized in LVAD activation and cardiopulmonary bypass weaning – inter-atrial and interventricular septal position along with chamber size and function identify early RV dysfunction and guide LVAD speed adjustments. Cameli and colleagues (5) highlight the importance of TOE in post implantation parameters such as aortic valve (AV) opening and complications such as cardiac tamponade, RV dysfunction, aortic regurgitation and thrombosis that are frequently detected by TOE. The highlighted impact on pre-LVAD hemodynamics, which may delay detection of entities such as tamponade without a high clinical degree of suspicion, has been well surmised.
The authors discuss AV opening, thrombosis and regurgitation in detail, highlighting the role of echocardiography and potentially computed tomography (CT). AV opening serves to prevent root thrombosis, however regurgitation may create a drop in the LVAD-aortic circulation resulting in decreased systemic circulation. AV opening utilizing transthoracic echocardiography (TTE)/TOE M-mode imaging through the long- axis of the aortic valve can predict eventual complications such as thrombosis and is specific for different LVADs
CT imaging is primarily useful in definition of thoracic anatomy. Coronary CT angiography (CCTA) can exclude coronary artery disease in heart failure patients at low to moderate pretest probability prior to LVAD implantation. Cardiac CT with delayed acquisition sequences have high sensitivity and specificity for detection of left atrium and left atrial appendage thrombus and is considered as acceptable alternate to TOE. Morphological evaluation of cardiac structures remains a strength of CT, with contrast and radiation exposure serving as primary limitations. There Is a need for further research to define specific CT parameters with prognostic benefit.
Device infection is a common complication after LVAD implantation, this can involve any part of the LVAD circuit however LVAD drive line is frequently involved (10-35% of all cases), especially at the entry site into the skin (12). TOE has a high sensitivity and specificity at detection of LVAD infection, however CT and fluorodeoxyglucose (FDG)-positron emission tomography (PET)/CT are increasingly useful. FDG-PET/CT has an overall sensitivity and specificity of 92 and 83% respectively at localizing device infection and internal extension (13).
The current evidence for the role of cardiac magnetic resonance imaging (cMRI) is limited, however it is valuable in patients with sub-optimal echocardiographic evaluation or when a more detailed assessment if needed (14). Late gadolinium enhancement (LGE) is the reference standard for detection of myocardial infarct and focal fibrosis. cMRI can help identify inflammatory heart disease, intracardiac thrombi and tissue characterization can identify myocardial edema and fibrosis.
Multimodality imaging enables clinicians to understand tools available to comprehensively evaluate LVAD implantation, function and prevent adverse events. Improvement in software intelligence, introduction of machine learning algorithms will almost certainly rapidly improve our ability to enhance outcomes of not just LVAD recipients but almost all heart failure patients.
Cameli et al. (5) have created a detailed yet easy-to-read review of current cardiovascular imaging parameters and their utility in clinical practice.
Peer-review: Internal
Conflict-of-interest: None to declare
Authorship: A.S.
Acknowledgement and Funding: None to declare
Statement on A.I.-assisted technologies use: The author did not use AI technology in preparing the manuscript
Availability of data and materials: Not applicable
References
| 1. Tedford RJ, Leacche M, Lorts A, Drakos SG, Pagani FD, Cowger J. Durable mechanical circulatory support: JACC Scientific Statement. J Am Coll Cardiol 2023; 82: 1464-81. doi: 10.1016/j.jacc.2023.07.019 https://doi.org/10.1016/j.jacc.2023.07.019 PMid:37758441 |
||||
| 2. Mehra MR, Uriel N, Naka Y, Cleveland JC Jr, Yuzefpolskaya M, Salerno CT, et al; MOMENTUM 3 Investigators. A fully magnetically levitated left ventricular assist device - final report. N Engl J Med 2019; 380: 1618-27. doi: 10.1056/NEJMoa1900486 https://doi.org/10.1056/NEJMoa1900486 PMid:30883052 |
||||
| 3. Stainback RF, Estep JD, Agler DA, Birks EJ, Bremer M, Hung J, et al; American Society of Echocardiography. Echocardiography in the management of patients with left ventricular assist devices: Recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr 2015; 28: 853-909. doi: 10.1016/j.echo.2015.05.008 https://doi.org/10.1016/j.echo.2015.05.008 PMid:26239899 |
||||
| 4. Sciaccaluga C, Soliman-Aboumarie H, Sisti N, Mandoli GE, Cameli P, Bigio E, et al. Echocardiography for left ventricular assist device implantation and evaluation: an indispensable tool. Heart Fail Rev 2022; 27: 891-902. doi: 10.1007/s10741-021-10073-1 https://doi.org/10.1007/s10741-021-10073-1 PMid:33428013 |
||||
| 5. Cameli M, Aboumarie HS, Pastore MC, Caliskan K, Cikes M, Garbi M,et al; Members of the 2022-2024 EACVI Scientific Documents CommitTOE. Multimodality imaging for the evaluation and management of patients with long-term (durable) left ventricular assist devices. Eur Heart J Cardiovasc Imaging 2024; 25: e217-e40. doi: 10.1093/ehjci/jeae165 https://doi.org/10.1093/ehjci/jeae165 PMid:38965039 |
||||
| 6. Neyer J, Arsanjani R, Moriguchi J, Siegel R, Kobashigawa J. Echocardiographic parameters associated with right ventricular failure after left ventricular assist device: A review. J Heart Lung Transplant 2016; 35: 283-93. doi: 10.1016/j.healun.2015.12.018 https://doi.org/10.1016/j.healun.2015.12.018 PMid:26856675 |
||||
| 7. Guendouz S, Rappeneau S, Nahum J, Dubois-Randé JL, Gueret P, Monin JL, et al. Prognostic significance and normal values of 2D strain to assess right ventricular systolic function in chronic heart failure. Circ J 2012; 76: 127-36. doi: 10.1253/circj.cj-11-0778 https://doi.org/10.1253/circj.CJ-11-0778 PMid:22033348 |
||||
| 8. Cameli M, Lisi M, Righini FM, Tsioulpas C, Bernazzali S, Maccherini M, et al. Right ventricular longitudinal strain correlates well with right ventricular stroke work index in patients with advanced heart failure referred for heart transplantation. J Card Fail 2012; 18: 208-15. doi: 10.1016/j.cardfail.2011.12.002 https://doi.org/10.1016/j.cardfail.2011.12.002 PMid:22385941 |
||||
| 9. Stricagnoli M, Sciaccaluga C, Mandoli GE, Rizzo L, Sisti N, Aboumarie HS, et al. Clinical, echocardiographic and hemodynamic predictors of right heart failure after LVAD placement. Int J Cardiovasc Imaging 2022; 38: 561-70. doi: 10.1007/s10554-021-02433-7 https://doi.org/10.1007/s10554-021-02433-7 PMid:34661853 PMCid:PMC8926966 |
||||
| 10. Jones N, Burns AT, Prior DL. Echocardiographic assessment of the right ventricle-state of the art. Heart Lung Circ 2019;28(9):1339-1350. doi: 10.1016/j.hlc.2019.04.016 https://doi.org/10.1016/j.hlc.2019.04.016 PMid:31175016 |
||||
| 11. Tamborini G, Muratori M, Brusoni D, Celeste F, Maffessanti F, Caiani EG, et al. Is right ventricular systolic function reduced after cardiac surgery? A two- and three-dimensional echocardiographic study. Eur J Echocardiogr 2009; 10: 630-4. doi: 10.1093/ejechocard/jep015 https://doi.org/10.1093/ejechocard/jep015 PMid:19252190 |
||||
| 12. Li X, Kondray V, Tavri S, Ruhparwar A, Azeze S, Dey A, et al. Role of imaging in diagnosis and management of left ventricular assist device complications. Int J Cardiovasc Imaging 2019; 35: 1365-77. doi: 10.1007/s10554-019-01562-4 https://doi.org/10.1007/s10554-019-01562-4 PMid:30830527 |
||||
| 13. Tam MC, Patel VN, Weinberg RL, Hulten EA, Aaronson KD, Pagani FD, et al. Diagnostic accuracy of FDG PET/CT in suspected LVAD infections: A case series, systematic review, and meta-analysis. JACC Cardiovasc Imaging 2020; 13: 1191-202. doi: 10.1016/j.jcmg.2019.04.024 https://doi.org/10.1016/j.jcmg.2019.04.024 PMid:31326483 PMCid:PMC6980257 |
||||
| 14. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al; ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2022; 24: 4-131. doi: 10.1002/ejhf.2333 https://doi.org/10.1002/ejhf.2333 PMid:35083827 |
||||
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

