AHA 2025 guidelines for acute coronary syndromes: latest evidence and comparison with ESC guidelines
EDITORIALS
AHA 2025 guidelines for acute coronary syndromes: latest evidence and comparison with ESC guidelines
Article Summary
- DOI: 10.24969/hvt.2025.597
- CARDIOVASCULAR DISEASES
- Published: 14/09/2025
- Received: 08/09/2025
- Accepted: 08/09/2025
- Views: 34631
- Downloads: 5974
- Keywords: Acute coronary syndrome, guidelines, STEMI, NSTE-ACS, risk stratification, management, revascularization, prevention
Address for Correspondence: Dario Mafrica, Department of Clinical, Internal Medicine, Anesthesiology and Cardiovascular Sciences, Sapienza University of Rome, Rome, Italy
E-mail: dariomafrica@gmail.com
ORCID: Dario Mafrica – 0009-0002-8991-1697; Giuseppe Biondi-Zoccai - 0000-00016103-8510; Marco Bernardi – 0000-0001-9269-8829
Dario Mafrica1, Giuseppe Franculli1, Antonio Rossi1, Antonio Esposito2, Giuseppe Biondi-Zoccai3,4, Marco Bernardi3, Pierre Sabouret5,6
1Department of Clinical, Internal Medicine, Anesthesiology and Cardiovascular Sciences, Sapienza University of Rome, Rome, Italy
2ICOT Istituto Marco Pasquali, Latina, Italy
3Department of Medical-Surgical Sciences and Biotechnologies, Sapienza University of Rome, Latina, Italy
4 Maria Cecilia Hospital, GVM Care & Research, Cotignola, Italy
5 Heart Institute and Action Group, Pitié-Salpétrière, Sorbonne University, Paris, France
6 National College of French Cardiologists, Paris, France
Abstract
The recently released 2025 ACC/AHA guidelines for the management of acute coronary syndromes (ACS) arise from the need to unify and update all contemporary evidence regarding the diagnosis, risk stratification, and treatment of ACS.
The document addresses both out-of-hospital management and in-hospital care, providing dedicated flowcharts for patients with ST-segment elevation myocardial infarction (STEMI)—where the primary goal is immediate reperfusion—and for those with non–ST-segment elevation ACS (NSTE-ACS), in whom therapeutic decisions depend on allocation into four distinct risk categories based on clinical presentation, hemodynamic status, and arrhythmic profile, supported by validated prognostic scores. The risk class determines the optimal timing of the invasive strategy.
A central role is attributed to antithrombotic therapy, encompassing both antiplatelet and anticoagulant agents, with strong emphasis on an individualized, patient-tailored balance between ischemic and bleeding risks to guide drug selection and treatment duration. Guideline further addresses secondary prevention, highlighting lipid-lowering strategies and evidence-based use of cardioprotective drugs.
Dedicated sections are devoted to the management of mechanical and electrical complications, cardiogenic shock, and advanced catheterization laboratory strategies, including complete revascularization in multivessel disease.
In our editorial, we provide a comparative analysis with the 2023 ESC Guidelines on ACS, underscoring that while the core principles remain largely concordant, subtle differences in clinical approach persist between the American and European documents.
Key words: Acute coronary syndrome, guidelines, STEMI, NSTE-ACS, risk stratification, management, revascularization, prevention
The recently released 2025 ACC/AHA guideline for the management of acute coronary syndromes (ACS) arise from the need to unify and update all contemporary evidence regarding the diagnosis, risk stratification, and treatment of ACS (1).
In our editorial, we provide a comparative analysis of 2025 ACC/AHA guideline for the management of ACS with the 2023 ESC guidelines on ACS (2), underscoring that while the core principles remain largely concordant, subtle differences in clinical approach persist between the American and European documents.
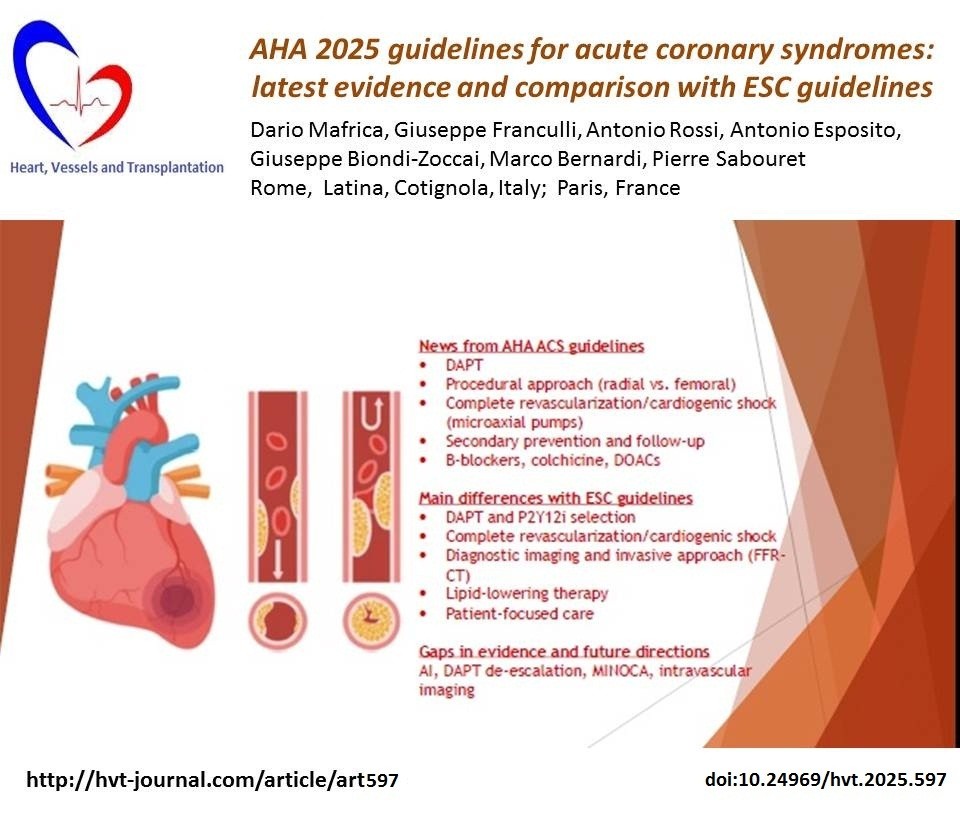
News from ACC/AHA 2025 ACS guidelines (Fig.1)
Dual antiplatelet therapy
According to the latest guidelines (1), in non-ST-elevation- acute coronary syndrome (NSTE-ACS) and ST-elevation- acute coronary syndrome (STE-ACS) patients undergoing percutaneous coronary intervention (PCI), a second P2Y12 inhibitor in addition to acetylsalicylic acid (ASA) , such as prasugrel or ticagrelor (if the invasive evaluation is unplanned, meaning that the coronary anatomy is not previously known) are recommended over clopidogrel (unless these two are contraindicated/not tolerated). Dual antiplatelet therapy (DAPT) therapy must be continued, if not contraindicated, for at least 1 year (IA).
2025 ACC/AHA guidelines tried to overcome the issues of the DAPT for those patients with lower ischemic risk to reduce the bleeding risk. In fact, after at least 1 month of DAPT it is possible to switch to ticagrelor monotherapy (IA) or to clopidogrel/prasugrel monotherapy (2b B-R). Another strategy is DAPT de-escalation (switching from powerful P2Y12 inhibitors to clopidogrel) after one month (2b B-R).
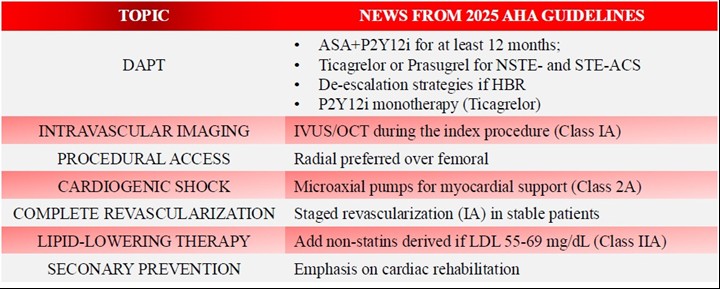
Figure 1. New recommendation in 2025 ACC/AHA guidelines
ASA- acetyl-salicylic acid, HBR – high bleeding risk, IVUS – intravascular ultrasound, oCT – optical coherence tomography, LDL – low-density lipoprotein cholesterol, NSTE –ACS – non-ST-elevation acute coronary syndrome, STE –ACS – ST-elevation acute coronary syndrome
For those patients who need oral anticoagulation (OAC), there are not significant differences from the previous guidelines. In these cases, it is suggested to undergo triple therapy (aspirin, clopidogrel and OAC) that has to be discontinued after 1 to 4 weeks (according to the hemorrhagic-ischemic risk balance) (IA), and after that, single antiplatelet therapy (clopidogrel is generally preferred over aspirin) + OAC therapy must be continued until one year, then suspended (IA).
The recently published randomized controlled Aquatic (3) study confirmed previous open label studies underlying that, even in French patients with chronic coronary syndrome and a higher ischemic risk than Asian patients, adding aspirin increases major bleeding and mortality without reduction of ischemic events.
Procedural approach
Transradial approach is generally preferred over femoral access because of its significantly lower association to all cause death and major bleeding. The MATRIX trial highlighted the lower association between the transradial approach and a lower rate of the endpoints of major adverse clinical events (MACE) and net adverse clinical events (death, myocardial infarction, and stroke in 1 month) (4).
Complete revascularization/cardiogenic shock
Cardiogenic shock is a relatively rare (almost 10%) complication of ACS, most of the times in STEMI, although it can be very severe, being associated with a high rate of early mortality (nearly 40-50%) (5).
To reduce cardiovascular mortality, several types of devices for mechanical circulatory support have been studied and developed especially in this setting:
1.Intraaortic balloon pump (IABP) improves coronary perfusion and reduces cardiac afterload. This counterpulsation improves coronary perfusion and reduces cardiac afterload. It is maybe the easiest devices to use and has a smaller rate of vascular access complication (6).
2.Percutaneous microaxial flow pumps ensure a continuous output as they drain blood from the left ventricle and release it into the ascending aorta. Those guarantee proper general perfusion but are also associated to a greater rate of vascular complications. The DanGer-SHOCK trial (7) has shown that the use of microaxial pumps significantly reduced risk of all-cause mortality at 6 months compared to the traditional standard of care, however being associated to an higher rate of complications like limb ischemia, bleeding and renal failure and replacement.
For those patients who received extracorporeal membrane oxygenation (ECMO) support for cardiogenic shock (CS) in ACS, the ECMO-CS trial showed no significant differences for all cause death between the group of patients that were rapidly deteriorating or with severe CS that underwent immediate venoarterial-ECMO and those who did not.
Secondary prevention/follow up
As for the follow-up of those patients, great importance is given to the cardiac rehabilitation (CR) program. CR is a complex and well organized “outpatient intervention”.
The main purposes of CR are modifying cardiovascular risk factors and to refine the global functional capacity of the patients. The CR is based on a set of things regarding a planned medication intake, a tailored health and nutritional training, and an overall improving of the quality of life (including a personal psychological support); This project, even thought has been shown to lower both the hospitalization rate and cardiovascular death, is underutilized, especially in females and in the underrepresented groups (8).
To overcome this, the new guidelines promote a centralized approach, so that the patients should be referred to an outpatient CR program prior to hospital discharge (IA); home based CR programs are also effective options (2a BR)
According to the 2025 ACC/ AHA guidelines, improving CR participation is a key priority (1).
However, it is underlined by guidelines itself that enrollments in rehabilitation programs are still low. The main issues with patients’ participation are represented by limited use of centralized referral systems via electronic health records, poor coordination among care teams, and patients’ perceptions of inconvenience and costs.
To overcome these problems and increase patients’ participation in CR programs, the latest guidelines propose early referral to CR, ideally during the index event, before hospital discharge. This system may allow not to “losing” the patient during the rehabilitation phase and programming adequate follow-up.
Other issues are represented by the difficulty in terms of access to care after hospitalization. On this side, AHA 2025 guidelines for ACS propose the use of home-based CR programs (1). Finally, for those patients who may accept and tolerate, guidelines mention the development of intensive CR programs, MACE.
Beta blockers; colchicine
Beta blockers are considered as a first option therapy as regards the acute myocardial infarction (MI) because of its effect in reducing the risk of reinfarction and both the onset and the recurrence of ventricular arrhythmias (9); and an early (<24h) initiation of this class of medications is recommended (I A). For those in which they are contraindicated (e.g. acute decompensated heart failure or high risk of an evolution to CS; II or III degree atrioventricular block; severe bradycardia; bronchospasm), are meant to be revalued after 24 hours to see if the initial contraindication has been solved.
Colchicine has been introduced as a potential preventive therapy, reducing the neutrophil adhesion to endothelial cells and platelets and the C-reactive protein. Actually in the ACC/AHA-ACS guidelines is considered as a COR 2b B-R, being associated with a lower risk of MI in patients with coronary artery disease (CAD), including those with prior MI, with contrasting results between randomized controlled trials (RCTs), advocating a personalized strategy (10).
Main differences with ESC guidelines
The main topics in which ESC and AHA guidelines differ are represented by the choice and the management of the DAPT, complete revascularization during the index procedure and CS, lipid-lowering therapy, advanced imaging techniques and patient-focus care (1, 2).
DAPT and P2Y12i selection
Antiplatelet therapy has always been a milestone in the management of CAD. Since the very acute phase, after the confirmed diagnosis of STEMI, both ESC and AHA guidelines suggest administering ASA load (class IA). The second antiplatelet agent load (a P2Y12 inhibitor) is still strongly recommended in ACC/AHA guidelines (class IA), while this recommendation has been de-escalated in ESC 2023 guidelines for ACS (class 2B). This de-escalation derived from the evidence of the studies ATLANTIC and SWEDEHEART registry (11, 12) and several meta-analysis, which demonstrated the absence of benefits in terms of mortality and adverse events in administering the P2Y12i load in the very acute phase (before coronary angiography).
For the prosecution of DAPT, the ACC/AHA guidelines are more rigid, suggesting continuing DAPT with potent P2Y12 inhibitors for at least 12 months (Class IIB) accepting a de-escalation with monotherapy with P2Y12i in patients with low ischemic risk after 3-6 months (Class IIA). On the other end, ESC guidelines introduced the possibility of monotherapy with P2Y12i after 1 month (Class IA).
Complete revascularization and cardiogenic shock
ESC and AHA guidelines for ACS have dealt with the topic of ACS presenting with multivessel disease and/or cardiogenic shock.
Both ESC and AHA guidelines strongly discourage revascularization of non-culprit lesions in cardiogenic shock (Class IIIA). This derives from the evidence of trials such as CULPRIT-SHOCK Trial (13), which demonstrated the higher rate of complications in those patients who have been subjected to complete revascularization, without a significant benefit in terms of long-term outcomes. Meanwhile, ACC/AHA guidelines suggest staged percutaneous coronary intervention (Class IIA) in post-shock phase, while ESC guidelines do not express opinion clearly about this topic.
Complete revascularization should be considered, in general, in selective procedures within 45 days since the index event (Class IA).
Lipid-lowering therapy
Both ESC and ACC/AHA stress the importance of lipid-lowering therapy for those patients who experienced an ACS, putting a target <55 mg/dL for low-density lipoprotein (LDL) cholesterol.
ACC/AHA guidelines tend to be more aggressive in terms of the usage of non-statin drugs such as ezetimibe and PCSK9 inhibitors such as alicrolumab, evelocumab and inclisiran if LDL levels are above 70 mg/dL at the time of the event (Class I) or between 55 and 69 (Class IIA).
The recent update in EAS/ESC guidelines has upgraded the “upfront strategy”, advocating the use of high-intensity statins and ezetimibe before discharge with the addition in some cases of bempedoic acid. The lipid check should be performed early after discharge (one month) to determine those eligible to PCSK inhibitors. The icosapent ethyl is now indicated at 2x2 grams daily in patients with triglyceride level above 135 mg/dL.
Advanced imaging and computerized tomography
Cardiac computed tomography (CT) angiography finds its place in ACC/AHA guidelines for ACS in the context of NSTE-ACS with low-to-intermediate risk, in those patients in which a selective strategy has been chosen, for a noninvasive risk stratification. This evidence comes from studies such as the VERDICT trial (14) which demonstrated the non-superiority of an early invasive approach in these patients vs. a selective approach.
On the other end, ESC guidelines tend to be more conservative and CT is indicated only in low-risk suspected ACS with negative troponins and negative electrocardiogram (ECG).
Patient-focused care
ACC/AHA guidelines tend to be more focused on procedural aspects, while ESC guidelines focus more on the multidisciplinary approach, integrating aspects such as multimorbidity, frailty, bleeding risk of the patient.
Gaps in evidence and future directions
In both 2023 ESC and 2025 ACC/AHA guidelines on ACS, several areas remain characterized by limited evidence, leading to cautious or weak recommendations.
Role of artificial intelligence in diagnostic evaluation for ACS
The 2023 ESC and 2025 ACC/AHA guidelines fail to provide recommendations for the clinical use of artificial intelligence (AI), highlighting a significant evidence gap. Prospective RCTs are needed to confirm AI's efficacy and cost-effectiveness. Although early results are promising, with deep learning algorithms outperforming clinicians and conventional software in ECG diagnosis (15), and AI enabling accurate quantitative and qualitative evaluation of plaque with coronary CT angiography (16) or invasive imaging such as optical coherence tomography (OCT) (17), multicenter validation and standardization are essential before integrating AI into guidelines.
DAPT de-escalation
The 2023 ESC and 2025 ACC/AHA guidelines recognize DAPT de-escalation only after the first month, with weak recommendations (Class IIb), due to several evidence gaps. There are not sufficient RCTs on early switching (<30 days) and studies performed in fragile populations (elderly, with chronic kidney disease, patients on OAC). The superiority of guided de-escalation strategies over unguided approaches remains uncertain, and comparative data with P2Y12 monotherapy are limited.
Mechanical circulatory support
The routine use of devices such as IABP or percutaneous ventricular assist systems is not recommended, except in selected patients with refractory CS.
However, major uncertainties persist regarding the optimal device, best timing for implantation, escalation and weaning strategies, and the true impact on mortality and secondary organ damage (renal, neurological).
The IABP-SHOCK II trial failed to show survival benefit of IABP, while the DANGER-Shock trial suggested improved outcomes with early Impella support in patients with acute MI complicated by shock, highlighting the evolving and controversial nature of this field (18, 19).
Intensive lipid-lowering therapy
Both ESC and AHA guidelines emphasize achieving stringent LDL-cholesterol targets with early initiation of high-intensity statins. However, solid evidence is lacking on the clinical benefit of “fast track” in-hospital initiation of ezetimibe or PCSK9 inhibitors. Trials like EVOPACS and PACMAN-AMI (20, 21) suggest potential advantages of early PCSK9 use in ACS, but larger dedicated studies are still needed. Moreover, the role and timing of inclisiran in the acute phase of ACS remain unexplored. Despite the proven biological efficacy and clinical safety of Inclisiran in several ORION studies, Inclisiran is not yet considered in ACS patients. The ongoing RCT ORION IV and VICTORION 2P will provide definitive answers on the magnitude of clinical benefits of Inclisiran in this setting.
Intravascular imaging (IVUS and OCT)
IVUS and OCT-guided PCI lead to optimized angioplasties, but supporting evidence derives from studies not specifically designed in the acute setting. Key gaps concern their impact on endpoints such as mortality and MACE, the identification of subgroups who could mostly benefit (e.g., high thrombus burden, specific lesion features), the role of deferred stenting strategies in plaque erosion, and the cost-effectiveness of routine implementation. The ULTIMATE trial supports IVUS-guided stenting, but ACS-specific RCTs remain limited (22).
MINOCA (myocardial infarction no obstructive coronary artery diseases) and SCAD (spontaneous coronary artery dissection)
Guidance is largely based on observational data, lacking RCTs to define the type and duration of antithrombotic therapy, the role of statins (restricted to patients with underlying atherosclerosis), or the benefit of beta-blockers and angiotensin-converting enzyme inhibitors. In SCAD, specific uncertainties concern patient selection for PCI, management during pregnancy, recurrence risk stratification, and long-term follow-up strategies.
Moreover, registries highlight that magnetic resonance imaging is still underused for the diagnosis of MINOCA due to several barriers (23).
Conclusions
The latest ACC/AHA guidelines for ACS brought up some news principally for the management of DAPT in patients who suffered from an ACS, pre-treatment, management of cardiogenic shock, multivessel disease, usage of imaging techniques beyond invasive coronary angiography for risk stratification, and long-term medical therapy. Nevertheless, further evidence from larger RCTs is needed, especially to improve diagnostic processes including AI-derived algorithms and to extend DAPT de-escalation options in higher risk patients. Finally, for those patients who experienced an ACS is needed an improvement for the access to secondary prevention and follow-up programs, such as cardiac rehabilitation.
Peer-review: Internal
Conflict of interest: None to declare
Authorship: D.M., G.F., A.R., A.E., G.B-Z, M.B., and P.S. equally contributed to preparation of manuscript and fulfilled authorship criteria
Acknowledgements and funding: None to declare
Statement on A.I.-assisted technologies use: We declare use AI-assisted technologies in preparation of this manuscript
Data and material availability: Does not apply
References
| 1. Rao SV, O'Donoghue ML, Ruel M, Rab T, Tamis-Holland JE, Alexander JH, et al. 2025 ACC/AHA/ACEPT/NAEMSP/SCAI Guideline for the management of pateints with acute coronary syndromes: A report from the American College of Cardiology/ American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2025; 131: e771-e862. | ||||
| 2.Byrne RA, Rosellio X, Coughlan JJ, Barbato E, Berry C, Chieffo A, et al. 2023 ESC guidelines for the management of acute coronary syndromes: Developed by the task force on management of acute coronary syndromes of the European Society of Cardiology. Eur Heart J 2023; 44: 3720-826. https://doi.org/10.1093/eurheartj/ehad191 PMid:37622654 |
||||
| Lemesle C, Didier R, Steg PG, Simon T, Montalescot G, Danchin N, et al. Aspirin in patients with chronic coronary syndrome receiving oral anticoagulation. New Engl J Med 2025; doi: 10.1056/NEJMoa2507532 https://doi.org/10.1056/NEJMoa2507532 PMid:40888725 |
||||
| 4. Valgimigli M, et al. Radial versus femoral access in patients with acute coronary syndromes undergoing invasive management: a randomised multicentre trial. Lancet 2015; 385: 2465-76, doi: 10.1016/S0140-6736(15)60292-6 https://doi.org/10.1016/S0140-6736(15)60292-6 PMid:25791214 |
||||
| 5. Helgestad OKLM et al. Temporal trends in incidence and patient characteristics in cardiogenic shock following acute myocardial infarction from 2010 to 2017: a Danish cohort study. Eur J Heart Fail 2019; 21: 1370-8. doi: 10.1002/ejhf.1566 https://doi.org/10.1002/ejhf.1566 PMid:31339222 |
||||
| 6.Miller PE, et al. Clinical outcomes and cost associated with an intravascular microaxial left ventricular assist device vs intra-aortic balloon pump in patients presenting with acute myocardial infarction complicated by cardiogenic shock. JAMA Intern Med 2022; 182: 926. doi: 10.1001/jamainternmed.2022.2735 https://doi.org/10.1001/jamainternmed.2022.2735 PMid:35849410 PMCid:PMC9295019 |
||||
| 7.Moller JE, Engstrom T, Jensen LO, Eskjaer H, Mangner N, Polzin A, et al. Microaxial flow pump or standard care in infract-related cardiogenic shock. New Engl J Med 2024; 390: 1382-93. https://doi.org/10.1056/NEJMoa2312572 PMid:38587239 |
||||
| 8. Ritchey MD, et al. Tracking cardiac rehabilitation participation and completion among medicare beneficiaries to inform the efforts of a national initiative. Circ Cardiovasc Qual Outcomes 2020; 13: doi: 10.1161/CIRCOUTCOMES.119.005902 https://doi.org/10.1161/CIRCOUTCOMES.119.005902 PMid:31931615 PMCid:PMC8091573 |
||||
| 9.Early intravenous then oral metoprolol in 45 852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 2005; 366: 1622-32. doi: 10.1016/S0140-6736(05)67661-1 https://doi.org/10.1016/S0140-6736(05)67661-1 PMid:16271643 |
||||
| 10.Nidorf SM,Eikelboom JW, Budgeon CA, Thompson PL. Low-dose colchicine for secondary prevention of cardiovascular disease. J Am Coll Cardiol 2013; 61: 404-10. doi: 10.1016/j.jacc.2012.10.027 https://doi.org/10.1016/j.jacc.2012.10.027 PMid:23265346 |
||||
| 11. Figtree GA, et al. Mortality and cardiovascular outcomes in patients presenting with non-st elevation myocardial infarction despite no standard modifiable risk factors: Results from the SWEDEHEART Registry. J Am Heart Assoc 2022; 15: 10.1161/JAHA.121.024818. https://doi.org/10.1161/JAHA.121.024818 PMid:35876409 PMCid:PMC9375489 |
||||
| 12. Montalescot G, et al. Ambulance or in-catheterization laboratory administration of ticagrelor for primary percutaneous coronary intervention for ST-segment elevation myocardial infarction: Rationale and design of the randomized, double-blind Administration of Ticagrelor in the cath Lab or in the Ambulance for New ST elevation myocardial Infarction to open the Coronary artery (ATLANTIC) study. Am Heart J 2013; 165: 515-22. doi: 10.1016/j.ahj.2012.12.015 https://doi.org/10.1016/j.ahj.2012.12.015 PMid:23537967 |
||||
| 13. Thiele H, Desch S. CULPRIT-SHOCK (Culprit Lesion Only PCI Versus Multivessel Percutaneous Coronary Intervention in Cardiogenic Shock). Circulation 2018; 137: 1314-6. doi: 10.1161/CIRCULATIONAHA.117.032907 https://doi.org/10.1161/CIRCULATIONAHA.117.032907 PMid:29581361 |
||||
| 14.Kofoed KF, et al. Early versus standard care invasive examination and treatment of patients with non-ST-segment elevation acute coronary syndrome. Circulation 2018; 138: 2741-50. doi: 10.1161/circulationaha.118.037152 https://doi.org/10.1161/CIRCULATIONAHA.118.037152 PMid:30565996 |
||||
| 15. Al-Zaiti s, et al. Machine learning-based prediction of acute coronary syndrome using only the pre-hospital 12-lead electrocardiogram. Nat Commun 2020; 11: 3966. doi: 10.1038/s41467-020-17804-2 https://doi.org/10.1038/s41467-020-17804-2 PMid:32769990 PMCid:PMC7414145 |
||||
| 16.Ebrahimihoor E, Lima JAC. Breaking the mold: embracing early detection of coronary artery disease by coronary computed tomography angiography and the role of cumulative risk factor exposure. Eur Heart J Cardiovasc Imaging 2024; 25; 1083-4. doi: 10.1093/ehjci/jeae131 https://doi.org/10.1093/ehjci/jeae131 PMid:38758972 |
||||
| 17. Niioka H, et al. Automated diagnosis of optical coherence tomography imaging on plaque vulnerability and its relation to clinical outcomes in coronary artery disease. Sci Rep 2022; 12; 14067. doi: 10.1038/s41598-022-18473-5 https://doi.org/10.1038/s41598-022-18473-5 PMid:35982217 PMCid:PMC9388661 |
||||
| 18.H. Thiele H, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. New Engl J Med 2012; 367: 1287-96. doi: 10.1056/NEJMoa1208410 https://doi.org/10.1056/NEJMoa1208410 PMid:22920912 |
||||
| 19.Thiele H, Smalling RW, Schuler GC. Percutaneous left ventricular assist devices in acute myocardial infarction complicated by cardiogenic shock. Eur Heart J 2007; 28: 2057-63. doi: 10.1093/eurheartj/ehm191 https://doi.org/10.1093/eurheartj/ehm191 PMid:17586541 |
||||
| 20. Koskinas KC, et al. Evolocumab for early reduction of ldl cholesterol levels in patients with acute coronary syndromes (EVOPACS). J Am Coll Cardiol 2019; 74: 2452-62. doi: 10.1016/j.jacc.2019.08.010 https://doi.org/10.1016/j.jacc.2019.08.010 PMid:31479722 |
||||
| 21.Räber L, et al. Effect of alirocumab added to high-intensity statin therapy on coronary atherosclerosis in patients with acute myocardial infarction. JAMA 2022; 327: 1771. doi: 10.1001/jama.2022.5218 https://doi.org/10.1001/jama.2022.5218 PMid:35368058 PMCid:PMC8978048 |
||||
| 22. Gao X-F, et al. 3-Year Outcomes of the ULTIMATE trial comparing intravascular ultrasound versus angiography-guided drug-eluting stent implantation. JACC Cardiovasc Interv 2021; 14: 247-57. doi: 10.1016/j.jcin.2020.10.001 https://doi.org/10.1016/j.jcin.2020.10.001 PMid:33541535 |
||||
| 23. Dagrenat S, Douair A, Aperet A, Range G, Georegs JL,Nallett O, etal. Diagnostic value of cardiac magnetic resonance imaging for myocardila infarction with non-obstructive coronary arteries: The CRIMINAL prospective registry. Arch Cardiovasc Dis 2025; doi: 10.1016/j.acvd.2025.06.071 https://doi.org/10.1016/j.acvd.2025.06.071 PMid:40651879 |
||||
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

